3105
Exchangeable Proton Spectroscopy using RACETE-FLEX
Fabian Tobias Gutjahr1,2, Simon Mayer2, and Peter M Jakob2
1Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Experimental Physics 5, University Wuerzburg, Wuerzburg, Germany
1Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Experimental Physics 5, University Wuerzburg, Wuerzburg, Germany
Synopsis
RACETE-FLEX is a combination of the novel RACETE-method and the spectroscopic FLEX method. RACETE is a method for imaging chemical exchange with positive contrast with concurrent water suppression. Combining the RACETE approach with the FLEX frequency labeling strategy leads to a high sensitivity exchangeable proton spectroscopy method.
Introduction
The detection of exchangeable protons has received a lot of attention in recent years, as it allows to probe for chemical compounds or metabolites related to physiological and pathological conditions1. The gold standard method CEST2 is based on selective saturation of exchangeable protons which leads to a reduction of signal strength in a subsequent MRI experiments. By varying the the frequency of the saturation pulses, spectra of exchanging sites can be acquired. FLEX3 is a more recent method for the generation of such spectra. As in CEST, FLEX reduces the signal magnitude, however spectral information is encoded using the chemical shift of the exchanging sites. Recently a new method for direct detection of exchangeable protons has been proposed4. This RACETE-method generates a positive contrast instead of reducing the water signal. In this abstract the FLEX-strategy is applied to the RACETE-experiment.Methods
In Fig. 1 a simplified RACETE pulse diagram is shown. The stimulated echo pathway is exploited for any protons exchanging to the water pool during the time interval texch. All exchanging magnetization is labeled using the selection-gradient Gs. As the RACETE signal is a stimulated echo of the transferred excitation, the phase of the exchanging magnetization is retained in the signal. Additional to the phase imprint of the excitation pulse-pair a phase is accumulated due to the difference in frequency of the exchanging pool and of the excitation pulses. This is exploited in the RACETE-FLEX experiment by repeatedly acquiring RACETE signals for several τ1-interval lengths. The resulting complex signal can be directly converted to a spectrum using the Fourier transform.Experiments were performed on a 750 MHz tomograph (Bruker BioSpin, Ettlingen, Germany). The sample was egg white from a hen’s egg, as egg white has a high mobile protein content [5]. The interval τ1 was stepped from 3 ms to 13 ms in steps of 0.8 ms. As reference a CEST spectrum was acquired using a fast CEST method [6].
Results and Discussion
In Fig. 2 a) and b) the RACETE signal magnitude over the τ1-interval between the excitation pulses and the corresponding spectrum is shown. The protein peaks exhibit a good separation from the residual water peak. However not all peaks contributing to the CEST spectrum (Fig. 2 c)) are visible in the RACETE-FLEX spectrum. This can be explained by the narrow bandwidth of the excitation pulse (approximate response shown by the grey line in spectrum) and the different sensitivity dependence on exchange rates of the RACETE and CEST approach4.Conclusion
RACETE-FLEX is an interesting approach to exchangeable proton spectroscopy, as it combines some of the favorable attributes of FLEX, such as the high spectral resolution and adjustable filtering of exchange rates with the advantages of the RACETE approach such as phase retention and the ability to measure a positive signal instead of a small changes in a large water background signal.Acknowledgements
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF Projekt 01EO1504)References
- Van Zijl, P. C. M., & Yadav, N. N. (2011). Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? MRM, 65(4), 927–948.
- Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). JMR 2000;143:79–87
- Friedman JI, McMahon MT, Stivers JT, van Zijl PC. Indirect detec- tion of labile solute proton spectra via the water signal using fre- quency-labeled exchange (FLEX) transfer. JACS 2010;132: 1813–1815
- Gutjahr, F. T., Munz, E., & Jakob, P. M. (2019). Positive chemical exchange contrast in MRI using Refocused Acquisition of Chemical Exchange Transferred Excitations (RACETE). ZmedPhys, 29(2), 184–191.
- A., Zhou, J., Yan, K., & Zhu, H. (2012). A simple model for understanding the origin of the amide proton transfer MRI signal in tissue. Applied Magnetic Resonance, 42(3), 393–402.
- Döpfert, J., Zaiss, M., Witte, C., & Schröder, L. (2014). Ultrafast CEST imaging. JMR, 243, 47–53.
Figures
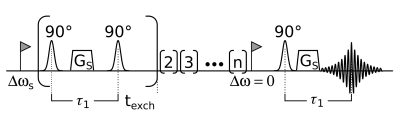
Simplified diagramm of the RACETE-sequence. By repeated excitation and storage of exchangeable magnetization a positive contrast stimulated echo is generated. The interval between the excitation pulses is τ1. Varying this interval enables the RACETE-FLEX experiment.
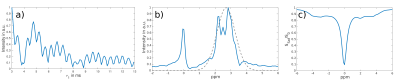
a) Magnitude of signal over time τ1 acquired in steps of 0.08 ms. b) corresponding RACETE-FLEX spectrum. The gray line shows the position and bandwidth of a single excitation pulse c) CEST spectrum (Preparation: 20 block pulses, 200 ms, 1 µT B1)