3096
Snapshot whole brain CEST MRI at 3T with 3D-EPI1High-field Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Department of Biomedical Magnetic Resonance, Eberhard Karls University Tuebingen, Tuebingen, Germany, 4Department of Physics and Astronomy, University of Bonn, Bonn, Germany, 5Department of Neuroradiology, University Hospital Erlangen, Erlangen, Germany
Synopsis
CEST MRI provides metabolite-based contrasts but often suffers from poor volume coverage or spatial resolution. We optimized and included a snapshot 3D-EPI readout and propose a suitable post-processing pipeline to generate CEST contrast in the whole brain at clinical B0=3T. It is shown that CEST MRI with 1.8mm isotropic nominal resolution at a field of view of 256x224x156mm³ is feasible within 4.3s per presaturation frequency offset. The approach is adaptable for any presaturation scheme. Exemplarily low power saturation was performed and fitted Lorentzian amplitudes gave a coefficient of variation <8.5% across three healthy subjects.
Introduction
CEST MRI provides contrast of low concentrated metabolites, proteins and is sensitive to external parameters such as pH value. To generate these contrasts, magnetization has to be prepared by applying a presaturation module at different frequency offsets. Since this prepared magnetization state will decay, CEST MRI requires a fast readout or has to be repeated even within the same saturation offset for segmented readout. Commonly, CEST MRI is therefore restricted to a relatively small field of view with few slices or poor spatial resolution. Therefore, we follow a recent approach at ultra-high field (1) optimizing a whole brain snapshot 3D-EPI readout (2) at a clinical 3T system. This approach overcomes the issue of limited spatial resolution and volume coverage in CEST MRI with no additional scan time.Methods
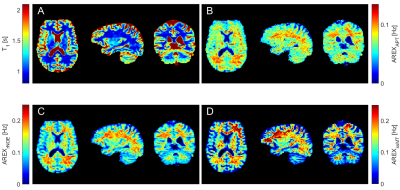
The 3D-EPI based protocol was optimized in five healthy subjects at a clinical 3T MR scanner (Prismafit, Siemens Healthineers, Erlangen, Germany) with the vendors 64Rx channel head/neck coil and body coil for transmit. Written informed consent was obtained from all participants prior to the examination. In the final protocol the imaging parameters were: nominal matrix size 144x126x88 ([RO=HF]x[PE=AP]x[3D=LR]) at 256x224x156mm³ field of view (CAIPIRINHA=1x6shift=2 along both PE directions; partial Fourier=6/8 in PE1; BW=1930Hz/pix; TE=11ms; EPI-factor=32; 45 binominal-11 water excitations per volume; FA=15°; centric reordering with half-elliptical scanning (2)). This resulted in a readout length of 1.2s for 1201 k-space lines at a nominal isotropic resolution of (1.8mm)³. CEST MRI was performed at three different B1 values with a total of four interleaved WASABI (3) scans. For presaturation 16x100ms Gaussian shaped pulses at 50% duty cycle were applied with recovery times of 0s and 12s for the unsaturated image (M0). Acquisition of each of the 57 frequency offsets took 4.3s plus one M0 image. This is less than 4:30min for a complete CEST spectrum. Additionally, T1 was estimated using a saturation recovery EPI sequence. Post-processing included motion correction using elastix (4), dynamic correction for B0 (5), Z-B1 correction (6) and denoising using principal component analysis (7). For CEST quantification a 4-pool Lorentzian model was fitted to the post-processed Z-spectra. With the acquired T1 maps AREX contrast (8) could be determined.Results
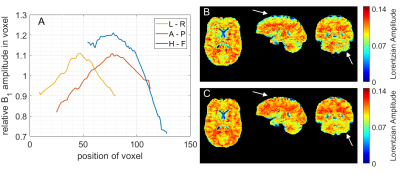
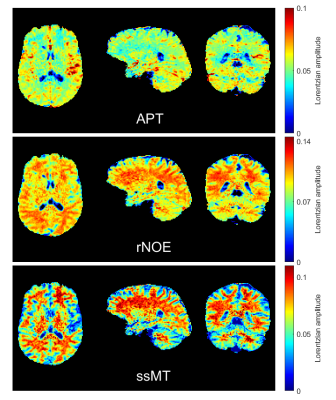
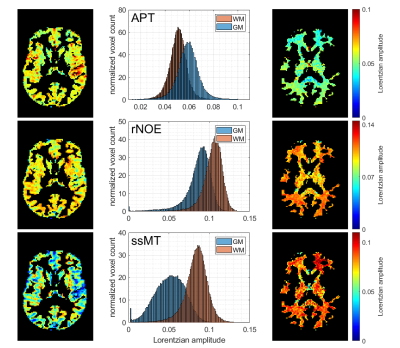
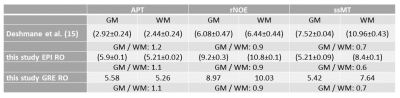
With the optimized protocol a tSNR of >75 for whole brain coverage with (1.8mm)³ even in the cerebellum was realized. Compared to an established 3D-GRE based protocol (9,10), at a resolution of (2.34mm)³, the EPI provided twice the tSNR. Figure 1 shows that B1 correction becomes necessary for whole brain coverage even at 3T. Z-B1 correction (6) with spline interpolation including three B1 values could correct for this issue. We found homogenous Lorentzian amplitudes across the whole brain volume with significant gray/white matter contrast for APT, rNOE and ssMT (Figure 2, Figure 3). Executing the suggested post processing pipeline, we achieved a coefficient of variation that was below 8.5 % for all fitted amplitudes, both in gray and white matter across three subjects (examined with final protocol, Table 1). The resulting AREX contrasts are shown in Figure 4.Discussion
To further reduce the overall acquisition time of <25min, the interleaved WASABI field mapping could be replaced by methods like DREAM(11)/3DREAM(12). Reducing time for field mapping to 1min each would save 24% of the entire acquisition duration. Since the 3D-EPI readout was realized as a snapshot approach, our protocol can be used for any CEST presaturation module or for example also for CEST MR-fingerprinting (13). With only 4.3s acquisition time per saturation offset and the high nominal resolution of (1.8mm)³ the approach is currently one of the best performing CEST MRI protocol at clinical field strength. With the large field of view and the high spatial resolution both spread out pathologies and smaller lesions across the whole brain might be investigated with CEST MRI at the same time.Conclusion
We believe that the suggested 3D-EPI-based CEST MRI acquisition and post-processing pipeline will enable a broad variety of applications that could bring CEST MRI further towards clinical routine.Acknowledgements
The financial support of the Max Planck Society, German Research Foundation (DFG, grant ZA 814/2-1), and European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 667510) is gratefully acknowledged.References
1. Akbey S, Ehses P, Stirnberg R, Zaiss M, Stöcker T. Whole-brain snapshot CEST imaging at 7 T using 3D-EPI. Magnetic Resonance in Medicine 2019;0 doi: 10.1002/mrm.27866.
2. Stirnberg R, Huijbers W, Brenner D, Poser BA, Breteler M, Stöcker T. Rapid whole-brain resting-state fMRI at 3 T: Efficiency-optimized three-dimensional EPI versus repetition time-matched simultaneous-multi-slice EPI. NeuroImage 2017;163:81–92 doi: 10.1016/j.neuroimage.2017.08.031.
3. Schuenke P, Windschuh J, Roeloffs V, Ladd ME, Bachert P, Zaiss M. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn Reson Med 2017;77:571–580 doi: 10.1002/mrm.26133.
4. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Transactions on Medical Imaging 2010;29:196–205 doi: 10.1109/TMI.2009.2035616.
5. Windschuh J, Zaiss M, Ehses P, Lee J-S, Jerschow A, Regatte RR. Assessment of frequency drift on CEST MRI and dynamic correction: application to gagCEST at 7 T. Magnetic Resonance in Medicine 2019;81:573–582 doi: 10.1002/mrm.27367.
6. Windschuh J, Zaiss M, Meissner J-E, et al. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR in Biomedicine 2015;28:529–537 doi: 10.1002/nbm.3283.
7. Breitling J, Deshmane A, Goerke S, et al. Adaptive denoising for chemical exchange saturation transfer MR imaging. NMR in Biomedicine 2019;0:e4133 doi: 10.1002/nbm.4133.
8. Zaiss M, Xu J, Goerke S, et al. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed 2014;27:240–252 doi: 10.1002/nbm.3054.
9. Zaiss M, Ehses P, Scheffler K. Snapshot‐CEST: Optimizing spiral‐centric‐reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR in Biomedicine 2018;31 doi: 10.1002/nbm.3879.
10. Mueller S, Deshmane A, Herz K, Scheffler K, Zaiss M. Development of whole-brain 3D snapshot CEST MRI at 3T. In: Proceedings of the International Society of Magnetic Resonance in Medicine. Vol. 27. ; 2019.
11. Nehrke K, Börnert P. DREAM—a novel approach for robust, ultrafast, multislice B1 mapping. Magnetic Resonance in Medicine 2012;68:1517–1526 doi: 10.1002/mrm.24158.
12. Ehses P, Brenner D, Stirnberg R, Pracht ED, Stöcker T. Whole-brain B1-mapping using three-dimensional DREAM. Magnetic Resonance in Medicine 2019;82:924–934 doi: 10.1002/mrm.27773.
13. Perlman O, Herz K, Zaiss M, Cohen O, Rosen MS, Farrar CT. CEST MR-Fingerprinting: practical considerations and insights for acquisition schedule design and improved reconstruction. arXiv:1904.09732 [physics] 2019.
14. Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851 doi: 10.1016/j.neuroimage.2005.02.018.
15. Deshmane A, Zaiss M, Lindig T, et al. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magnetic Resonance in Medicine 2018;0 doi: 10.1002/mrm.27569.
Figures