3095
Frequency-stabilized chemical exchange saturation transfer imaging with free induction decay readout1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, 2Department of Radiology, Children's Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China, 3MR Collaboration, Siemens Healthcare Ltd., Shanghai, China, 4Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China
Synopsis
CEST imaging is highly sensitive to temporal B0 drift, for which a frequency-stabilized CEST (FS-CEST) sequence was recently proposed by inserting a frequency stabilization module in front of the conventional non-frequency-stabilized CEST (NFS-CEST) sequence. Here, the frequency stabilization module in the FS-CEST sequence was further simplified by replacing the original gradient-echo readout with free induction decay (FID) readout. The proposed FS-CEST sequence with FID readout in the frequency stabilization module was validated in phantoms on a 3T Siemens Prisma scanner and in 15 volunteers on a 3T Philips Achieva scanner, both leading to improved CEST maps.
Introduction
Chemical exchange saturation transfer (CEST) imaging, a molecular imaging technique, is capable of indirectly detecting the low-concentration metabolites with water-exchangeable protons through frequency-selective saturation.1,2 However, CEST imaging is highly sensitive to temporal B0 drift, especially with magnetization transfer ratio asymmetry (MTRasym) analysis.3-5 The temporal B0 drift may result from the heating of ferromagnetic shim elements, which is typically encountered in echo planar imaging (EPI) sequences. Furthermore, the temporal B0 drift can persist for a substantial period after the EPI sequence 6,7 and cannot be easily corrected retrospectively for CEST imaging.8 Recently, a frequency-stabilized CEST (FS-CEST) sequence was proposed by inserting a frequency stabilization module in front of the conventional non-frequency-stabilized CEST (NFS-CEST) sequence to correct temporal B0 drift in real time.9 In this study, the frequency stabilization module in the FS-CEST sequence was further simplified by replacing the original gradient-echo (GRE) readout of three k-space lines with free induction decay (FID) readout of a single k-space line. A cross-vendor validation of the proposed FS-CEST sequence with FID readout was performed in phantoms and normal volunteers on 3T Siemens Prisma and Philips Achieva scanners.Theory
The sequence diagram of the proposed FS-CEST sequence with FID readout is shown in Fig. 1, which replaces GRE readout 9 by FID readout of a single k-space line in the frequency stabilization module. The FID frequency stabilization module commences with a small flip angle excitation pulse in conjunction with slice-selective gradients followed by FID readout and a spoiler gradient. The FID readout samples 256 points in k-space with a dwell time of Δt=25us. The acquired k-space line is divided into odd and even parts, leading to an effective echo time difference of Δt between the two parts. After taking conjugate multiplication of the two parts, the phase differences between these two parts, $$$\Delta \overline{\varphi}$$$,is obtained by averaging the phase difference of all points. The frequency drift can be then quantified from the phase difference as follow. $$\Delta f=\frac{\Delta \overline{\varphi}}{2\pi\cdot \Delta t }$$The calculated frequency drift, $$$\Delta f$$$, is used to update the main B0 frequency in the succeeding CEST saturation, fat suppression and data acquisition modules in real time.Methods
The phantom experiments were performed on a 3T Siemens Prisma scanner and in vivo experiments were carried out on a 3T Philips Achieva scanner. The phantom consisted of a flask filled with 2% agarose gel and two test tubes. One tube was filled with 10% bovine serum albumin (BSA) dissolved in phosphate-buffered saline (PBS), and the other one with 5% BSA dissolved in PBS. Because the phantom experiments were conducted on a lightly-used research-dedicated scanner, temporal B0 drift was introduced by shifting the B0 frequency by +1Hz or -1Hz cumulatively for each CEST frame, resulting in a total B0 drift of +62Hz or -62Hz throughout 63 CEST frames. The in vivo study was done on an intensively-used clinical scanner, with IRB approval and written parental consent forms obtained from all 15 human subjects (7.0±3.1 years old). For both phantom and human studies, all NFS-CEST, FS-CEST with GRE readout, and FS-CEST with FID readout sequences were run consecutively. The key imaging parameters used on both scanners were: saturation power=2uT, saturation duration=4x200ms, TR=3000ms, TE=6.7ms, slice thickness=5mm, FOV=212x186mm2, and resolution=2.2x2.2mm2.Results
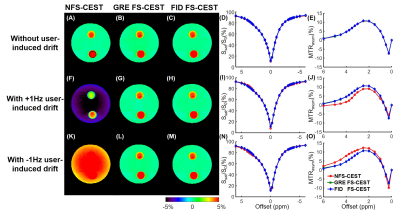
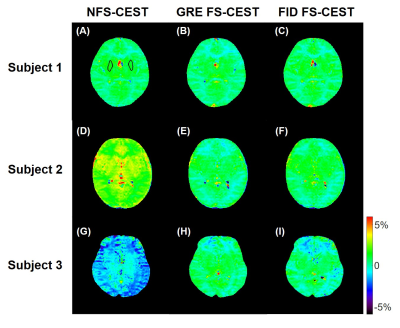
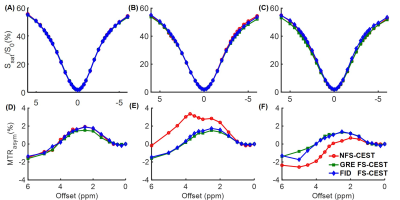
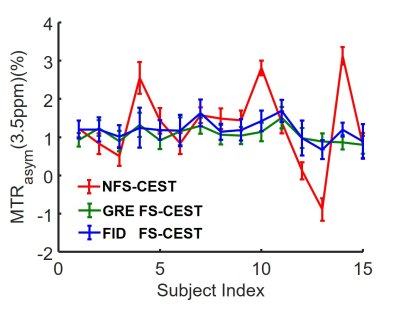
In the phantom study, the NFS-CEST sequence with positively- or negatively-induced B0 drift led to evidently abnormal amide proton transfer weighted (APTw) images and MTRasym spectra compared with those without drift. However, the FS-CEST sequences with both GRE and FID readout successfully corrected the user-induced B0 drift, generating consistent APTw images and MTRasym spectra with those without B0 drift (Fig. 2). In the in vivo study, APTw images obtained from NFS-CEST were greater than, smaller than or identical to those acquired from FS-CEST with GRE and FID readout (Fig. 3). In addition, little difference could be seen from the results between GRE FS-CEST and FID FS-CEST (Fig. 3). Furthermore, the human MTRasym spectra from NFS-CEST could be higher or lower than those from FS-CEST, but the MTRasym spectra from GRE FS-CEST and FID FS-CEST were highly consistent (Fig. 4), which agreed well with the phantom study. Lastly, Fig. 5 shows that the ROI-average APTw signals from all 15 volunteers using NFS-CEST had significant variability, while those using FS-CEST with GRE or FID readout were tightly bound.Discussion and Conclusion
We proposed a novel FS-CEST sequence with the acquisition of only one FID line in the frequency stabilization module to correct the temporal B0 drift in real time, which was validated against the previous FS-CEST approach with GRE readout in all experiments on cross-vendor scanners. Readout with a single FID line instead of three GRE lines further simplified the sequence design and reduced the minimal achievable duration of the frequency stabilization module. In conclusion, the proposed FS-CEST sequence with FID readout can correct the temporal B0 drift and generate more stable APTw images and MTRasym spectra than those from the NFS-CEST sequence on cross-vendor scanners.Acknowledgements
NSFC grant number: 61801421, 81971605. Zhejiang Lab grant number: 2018EB0ZX01. This work is also supported by “the Fundamental Research Funds for the Central Universities (2019FZJD005)”.References
1. Ward K, Aletras A, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000;143(1):79-87.
2. Van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011;65(4):927-948.
3. Zhou J, Blakeley JO, Hua J, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 2008;60(4):842-849.
4. Zu Z, Janve VA, Xu J, et al. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson Med 2013;69(3):637-647.
5. Zu Z, Xu J, Li H, et al. Imaging amide proton transfer and nuclear overhauser enhancement using chemical exchange rotation transfer (CERT). Magn Reson Med 2014;72(2):471-476.
6. Silva AC, Merkle H. Hardware considerations for functional magnetic resonance imaging. Concepts in Magnetic Resonance Part A: An Educational Journal 2003;16(1):35-49.
7. Foerster BU, Tomasi D, Caparelli EC. Magnetic field shift due to mechanical vibration in functional magnetic resonance imaging. Magn Reson Med 2005;54(5):1261-1267.
8. Windschuh J, Zaiss M, Ehses P, et al. Assessment of frequency drift on CEST MRI and dynamic correction: application to gagCEST at 7 T. Magn Reson Med 2019;81(1):573-582.
9. Liu R, Zhang H, Niu W, et al. Improved chemical exchange saturation transfer imaging with real‐time frequency drift correction. Magn Reson Med 2019;81(5):2915-2923.
Figures
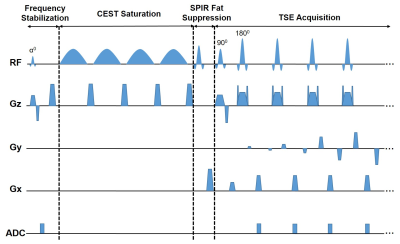
Figure 1. Schematic diagram of the proposed FS-CEST sequence with free induction decay (FID) readout. The FS-CEST sequence consists of four modules, including frequency stabilization, CEST saturation, SPIR fat suppression, and TSE acquisition. In the frequency stabilization module, the FID signals were read out after a small flip angle excitation and followed by a spoiler gradient.