3093
Protein-sensitive CEST-MRI of Alzheimer’s patients at 3 T1Divsion of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Center for Psychosocial Medicine, Department of General Psychiatry, Heidelberg University, Heidelberg, Germany, 3Neuroradiology, University of Erlangen-Nürnberg, Erlangen, Germany, 4Departement of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 5Departement of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
In this study, the potential of CEST-MRI as a tool for diagnostic imaging of Alzheimer’s patients was evaluated. Although the study size was yet too small to draw final conclusions, a significant decrease of the rNOE-CEST signal in Alzheimer’s patients was observed. This finding was in line with previous in vitro studies on the monitoring of protein aggregation using CEST-MRI. A diagnostic tool for Alzheimer’s based on CEST-MRI would allow avoiding invasive examinations and thus more frequent follow-ups allowing an improved level of patient care.
Introduction
Chemical exchange saturation transfer (CEST) MRI has already been shown to be sensitive to structural and conformational changes of proteins and peptides.1-3 In particular, we were able to monitor the aggregation of amyloid beta and the formation of amyloid fibrils in vitro by a decrease of the relayed nuclear Overhauser (rNOE) CEST signal.3 This finding led to the hypothesis that CEST-MRI allows the diagnostic imaging of patients with Alzheimer's disease where amyloidogenesis develops as a pathological hallmark. Due to sequestration of functional proteins into deposited aggregate plaques in Alzheimer’s disease we expected a decrease in the rNOE signal.3 Recently, this decrease in the rNOE signal was verified by an independent research group using a Alzheimer’s disease transgenic mouse model.4 However, the verification in humans still remains to be investigated, which is why, the aim of this study was to evaluate the potential of CEST-MRI for diagnostic imaging of Alzheimer’s patients.Methods
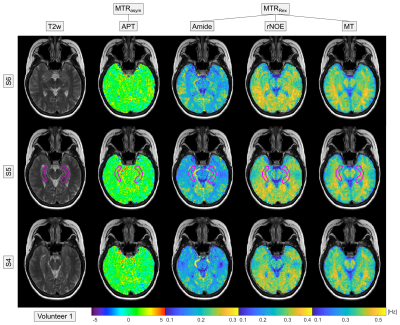
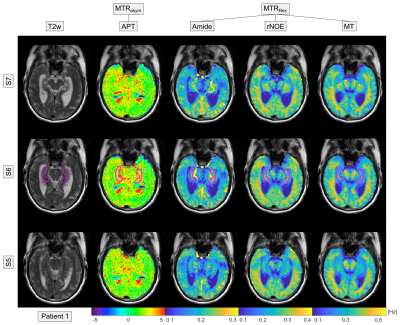
Four patients with diagnosed Alzheimer’s disease (66, 56, 52 and 78 years) and two healthy volunteers (56 and 57 years) were examined. Healthy controls were selected from the same age group being older than 50 years in age. Examinations were approved by the local ethics committee.All examinations were performed on a 3 T MR scanner (Siemens Prisma). CEST imaging of the isolated Amide, rNOE and semi-solid magnetization transfer (MT) signal was realized by a previously optimized acquisition protocol for relaxation-compensated 3D-CEST-MRI (B1 = 0.7 µT, 1.7×1.7×3 mm3, 12 slices).5 This comprises (i) an image registration to compensate motion-induced artifacts, (ii) a multi-pool Lorentzian-fit analysis to isolate the Amide, rNOE and MT signal, (iii) a relaxation compensation to evade spillover effects (MTRRex = 1/Z – 1/Zref), and (iv) a correction for B0- and B1-field inhomogeneities. For comparison, conventional amide proton transfer (APT) imaging (B1 = 2 µT) based on the asymmetry approach (MTRasym = Zref – Z) was performed using the established acquisition protocol of Zhou J, et al.6
Results
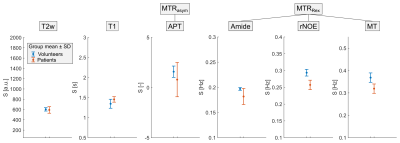
In healthy volunteers, the acquired multi-pool CEST images showed the expected contrasts: a hyperintense Amide signal in gray matter, hyperintense rNOE and MT signal in white matter, and a constant APT map throughout the whole brain (Figure 1). The acquisition of high-resolution 3D image data revealed also a hyperintense Amide signal in vessels (Figure 1 and 2, brain stem). In Alzheimer’s patients, a global decrease of the rNOE and MT signal was apparent indicating a considerable decline of the brain structure (cf. Figure 1 and 2). This was in coherence with the observed severely enlarged ventricles in Alzheimer’s patients. Statistical analysis of the different CEST contrasts in the central part of the Hippocampus (Figure 1 and 2, magenta line) revealed a significant decrease (with group mean values outside the error range) of the rNOE and MT signal in Alzheimer’s patients. For the rNOE and MT signal, the decrease was –12.4% and –13.4%, respectively. In contrast, no significant changes were apparent for the Amide, APT, T2w, and T1 signal.Discussion
The preliminary results support the hypothesis that CEST-MRI allows the diagnostic imaging of patients with Alzheimer's disease. As expected from our in vitro experiments3 and in coherence with the recent results in a mouse model,4 a significant decrease in the rNOE signal in Alzheimer’s disease was observed. In addition, the observed significant decrease of the MT signal offers the opportunity as a further marker for Alzheimer’s disease. Age-related biases were minimized by inclusion of subjects being from the same age group. Moreover, the insignificant changes in the T1 of water verify the origin of the observed rNOE signal decrease to be truly CEST-related. However, the study size (4 patients and 2 volunteers) is yet too small to check for statistical significance using e.g. a Student’s t-test. Consequently, final conclusions can only be drawn after inclusion of more study participants. In the future, also other brain regions besides the Hippocampus will be evaluated which is possible due to the acquired 3D image data.Conclusion
CEST-MRI has been demonstrated to be a promising tool for diagnostic imaging of patients with Alzheimer's disease. As curative treatments of Alzheimer’s are still unavailable, measures to slow down or even stop progression are of particular importance. For such measures, an early and precise diagnosis is critical which presently involves invasive examinations. A diagnostic tool based on the non‐invasive methodology of CEST-MRI would allow examinations on a more regular and frequent basis allowing progress monitoring and thus an improved level of patient care.Acknowledgements
We gratefully thank the German Research Foundation (DFG; GO 2172/1-1), the Helmholtz Association, and the Max Planck Society for the financial support. In addition, we cordially thank the medical-technical radiology assistants from the DKFZ for their help in conducting the examinations.References
1. Zaiss M, Kunz P, Goerke S, et al. MR imaging of protein folding in vitro employing Nuclear-Overhauser-mediated saturation transfer. NMR Biomed. 2013;26:1815-1822.
2. Goerke S, Zaiss M, Kunz P, et al. Signature of protein unfolding in chemical exchange saturation transfer imaging. NMR Biomed. 2015;30:906-913.
3. Goerke S, Milde K, Bukowiecki R, et al. Aggregation-induced changes in the chemical exchange saturation transfer (CEST) signals of proteins. NMR Biomed. 2017;30:e3665.
4. Chen L, Wei Z, Chan K, et al. Protein aggregation linked to Alzheimer's disease revealed by saturation transfer MRI. NeuroImage. 2019;188:380-390.
5. Goerke S, Soehngen Y, Deshmane A, et al. Relaxation‐compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn Reson Med. 2019;82:622-632.
6. Zhou J, Heo H-Y, Knutsson L, et al. APT-Weighted MRI: Techniques, Current Neuro Applications, And Challenging Issues. J Magn Reson Imaging. 2019;50:347-364.
Figures