3091
Ultrafast 3D Steady-State Chemical Exchange Saturation Transfer (CEST) MRI with Incoherent Sampling at 7T1Siemens healthineers Ltd, Seoul, Republic of Korea, 2Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon, Republic of Korea, 3. Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Republic of Korea
Synopsis
CEST MRI exploits saturation transfer-induced proton exchange and its corresponding, indirect loss of water signal, has been shown to provide a novel contrast mechanism in MRI. However, CEST MRI is prone to B0 inhomogeneities. To resolve this problem, CEST MRI requires multiple-acquisition. To acquire z-spectrum of whole brain at ultrahigh field of 7T, there are multiple issues to be tackled; a prohibitively long acquisition time, potential high power deposition, and B0 drift during a long scanning time. In this work, we proposed the rapid steady-state CEST MRI pulse sequence incorporating with incoherent sampling in 3D segmented EPI at 7T
Introduction
Chemical Exchange Saturation Transfer (CEST) MRI exploits saturation transfer-induced proton exchange and its corresponding, indirect loss of water signal [1-2]. Since multiple exchangeable protons and nuclear Overhauser effects contribute to CEST MRI, the separation of multiple CEST contributions to individual CEST contribution is useful. This requires to spread chemical shifts as wide as possible by using high magnetic fields, and to acquire multiple frequencies (z-spectrum) for the deconvolution of multiple components. Additionally, CEST is prone to B0 drift, causing serious artifacts. To acquire z-spectrum of whole brain at ultrahigh field of 7T, there are multiple issues to be tackled; a prohibitively long acquisition time, potential high power deposition, and B0 drift during a long scanning time. In this work, we proposed the rapid steady-state CEST MRI pulse sequence incorporating with incoherent sampling in 3D segmented EPI at 7T.Method
Rapid Steady-State 3D CEST MRI Pulse SequenceA sequence diagram of the proposed method is shown in Fig. 1. Gaussian-shaped RF pulse was applied for generating rapid steady-state CEST contrast, followed by spoiling gradient. The length of a non-selective rectangular RF pulse was adjusted to set fat resonance frequency to the zero-crossing offset frequency of the sinc-shaped excitation profile. This fat suppression scheme selectively excite water signals at ultra-high field 7T, resulting in shorter RF pulse duration and lower SAR than the conventional fat suppression method [3]. Finally, segmented 3D EPI acquisition was employed in the step of z-spectrum encoding with an incoherent compressed sensing (CS) sampling pattern.
Random Sampling for Segmented 3D-EPI Encoding
Conventionally, CS techniques require incoherent random sampling. However, implementation of incoherent random sampling to the 3D segmented EPI is sensitive to signal modulation due to changing blipped gradients. To tackle this problem, we proposed grouped random sampling approach (Fig. 2(a)). Thirteen k-space segments (see Group shot number in the kz direction in Fig. 2a) were used; in each segment, k-space sampling points were grouped along both kz and ky direction to restrict the maximum size of the blip gradient size In this specific case, each segment covered 8 kz and 29 ky points. To make signal modulation smoothly, encoding order of ky-segments matched with EPI factor of echoes. Then the central fully-sampled k-space lines were acquired at the end of the encoding order (in our case #13) to ensure steady-state of the CEST contrast. To maximize the CS performance, the sampling pattern was randomly varied along the frequency direction (see Fig.2 (b))
Experimental Validation
We used a 7T scanner (Magnetom Terra, Siemens Healthineers, Erlangen, Germany) with a 32-channel receive array head coil. CEST irradiation amplitude and duration were 0.7µT / 25ms. A total 51 images of human subjects were acquired at different saturation offset (±10, ±7, ±6, ±5.5, ±5, ±4.75, ±4.5, ±4.25, ±4, ±3.75, ±3.5, ±3.25, ±3, ±2.75, ±2.5, ±2.25, ±2, ±1.75, ±1.5, ±1.25, ±1, ±0.75, ±0.5, ±0.25, 0, ±300) relative to the water frequency. Other image parameters: FOV=210x173x230; matrix size=128x116x96 (kx,ky,kz); 1.8mm isotropic resolution; flip angle=10 degree; TR = 75ms, TE = 20ms, EPI factor =29, and total imaging time = 5min. All images were reconstructed using k-t sparse SENSE technique [4] and B0 correction was made with the WASSR technique [5].
Results and Discussion
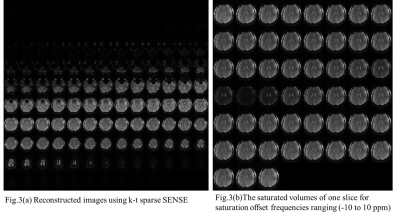
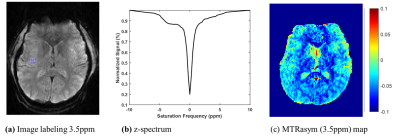
Fig. 3(a) shows (unsaturated) reconstructed images using k-t sparse SENSE. Acquired highly accelerated 3D segmented EPI shows excellent image quality without aliasing artifact. This indicates that sparse sampling and reconstruction produce high quality images with high temporal resolution. In Fig. 3(b), z-spectra images were shown for one selected slice. An image with direct water saturation (DS) effect near 0ppm shows at the middle of series of CEST images, indicating saturation is effectively achieved by steady-state saturation pulses. Clearly, 3-D CEST images were acquired rapidly without much artifacts. Fig. 4 represents feasibility of CEST effect using z-spectrum and MTR asymmetric method. As a result, the proposed method is highly effective in generating proton exchange induced image contrast at ultra-high field 7T.Conclusion
We successfully demonstrated that whole-brain CEST MRI can be acquired highly accelerated a novel steady-state 3D CEST pulse sequence potentially enabling whole brain CEST about 5min at ultra -high field 7T.Acknowledgements
No acknowledgement found.References
1. Guanshu Liu et al, NMR BIOMED 26: 810-828 (2013)
2. Peter C.M. van Zijl et al, MRM 65: 927-948 (2011)
3. Rudiger Stirnberg et al, MRM 76: 1517-1523 (2016)
4. Ricardo Otazo et al, MRM 64: 767-776
5. Mina Kim et al, MRM 61: 1441-1450 (2009)
Figures
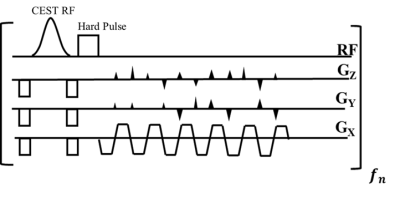
Fig.1 A schematic diagram of pulse sequence
The proposed rapid steady-state 3D CEST MRI pulse sequences with an efficient fat suppression method using rectangular RF pulse with 2041µs duration.
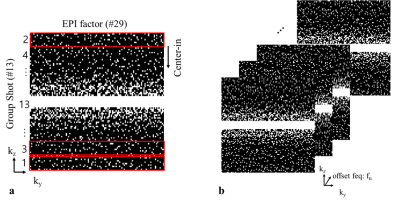
Fig.2 Proposed incoherent sampling pattern for segmented 3D EPI
(a)To reduce the blip size, kz-direction is segmented to 13 segments and separately acquired. The center segment is acquired at the last order to ensure steady state of the CEST contrast. The segmented k-space are acquired according to the EPI encoding. (b)These sampling pattern is randomly varied according the frequencies direction to maximize incoherence.