3090
On the feasibility of a single scan B1+ correction scheme in combination with Multiple Interleaved Mode Saturation for quantitative CEST at 7 Tesla1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Department of Neuroradiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 5Institute of Medical Physics, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
Synopsis
In Chemical Exchange Saturation Transfer MR both B1+-inhomogeneity correction and mitigation have their limitations, in particular if large field-of views shall be covered. To overcome these limitations a Multiple Interleaved Mode Saturation scheme was applied together with a linear B1+ inhomogeneity correction method. Repeatability and reproducibility of the MTRRex metric for rNOE and APT were investigated. A combination of MIMOSA and a simple single point correction allows achieving repeatable and reproducible CEST contrast in whole brain with an acquisition time of 4min 54s.
Introduction
Quantitative chemical exchange saturation transfer (CEST) MRI at ultra-high field (B0≥7 Tesla) requires correction1-3 or mitigation4,5 of the B1+ transmit (B1+) field inhomogeneity. Mitigation approaches either require an additional parallel transmission (pTx) pulse calculation4 or have smaller field-of-view in which the method is applicable as in case of Multiple Interleaved Mode Saturation(MIMOSA)5,6. On the other hand, correction methods usually require longer acquisition times2. Results by Akbey et al.7 indicate that the inverse magnetic transfer ratio metric (MTRRex) of relayed Nuclear Overhauser Effect (rNOE) and amide proton transfer (APT) can be corrected only in a certain region of interest without introducing major errors. This volume restriction is given by regions with high enough B1+ efficiency.In this work, we combine both, mitigation of B1+-inhomogeneity via MIMOSA5 and B1+ correction to enable whole brain CEST measurements with minimized B1+ inhomogeneity effects. In addition, the potential to shorten the acquisition by using a single point correction is evaluated. Both, reproducibility, i.e. how results with different B1+ correction schemes compare, and repeatability, i.e. how two measurements with same B1+ correction method compare, were investigated.
Materials & Methods
Data AcquisitionA 7-Tesla whole-body MR system (Magnetom Terra, Siemens Healthineers, Erlangen, Germany) with a 8Tx/32Rx head coil (Nova Medical, Wilmington, USA) was used for the measurements. In vivo measurements were performed on 2 healthy volunteers. Each volunteer was measured twice on the same day each time with a new positioning.
3D snapshot-CEST sequence8,9 was used to acquire the CEST images. MIMOSA was applied to achieve more homogenous saturation through interleaving of two sets of complimentary transmitter phases and constant amplitudes (‘modes’)5. B1+ maps of both modes were acquired using a pre-saturated 2D turbo-flash sequence10. B1+ distribution of MIMOSA was calculated based on the B1 continuous wave power equivalent (CWPE)11. Relative B1+ (rB1) maps were calculated by dividing the acquired maps through a nominal flip angle. Anatomical images were acquired with the use of an inhouse pTx-MPRAGE sequence.
Sequence parameters: CEST Saturation parameters: τp= 46.08ms, τD=30ms, n=50, Trec=1s, B1,Nominal=0.72, 0.96 and 1.08µT, Gaussian pulse shape; Gradient echo image acquisition parameters: FA=6°, TR=3.7ms, TE=1.6ms, matrix size=96x96x72, FoV=220x220x180mm3, GRAPPA 3x2; Total measurement time for a single CEST acquisition 4min 54s.
Data Analysis
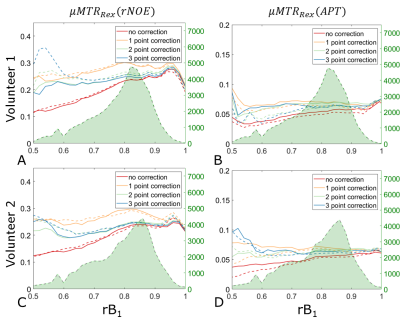
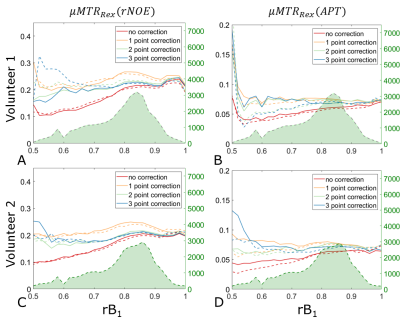
All acquisitions of a single volunteer were co-registered to the anatomical acquisition. All registrations were performed using SPM 1211. The anatomical images were segmented into gray (GM) and white matter (WM) with the use of the FSL FAST algorithm12. MTRRex(rNOE), MTRRex(APT)2 contrasts were calculated and corrected with a B1+ correction. The correction was performed with different numbers of acquisitions (Ncorr=1:3) correcting to a B1+ value of 0.72µT. Results were compared to MIMOSA without additional correction (Ncorr=0). To investigate the overall repeatability of the combination between MIMOSA and the B1+ correction, histograms of the MTRRex(rNOE) inside of the two chosen segments were investigated for each of the volunteers. Further on we investigated the reproducibility and repeatability of the B1+ correction methods in conjunction with MIMOSA. For this purpose, separately for each segment, the MTRRex metrics were binned based on the rB1,CWPE values and a mean value (µMTRRex) in each bin was calculated.
Results & Discussion
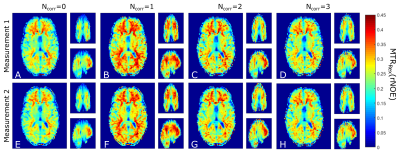
3D CEST acquisition with MIMOSA preparation pulses and without a B1+ correction shows a strong drop in the MTRRex values in the cranial part of the brain (Figure 1A,E). A difference in the resulting MTRRex values can be observed between the B1+ correction with Ncorr=1 and Ncorr=2,3. This is in agreement with previous results2,7. It should be noted that all acquisitions show high repeatability, both in WM and GM, as confirmed by histogram analysis (Figures 2A-D, 2E-H).The µMTRRex values still show a variation with rB1 (Figure 3, 4). This is in agreement with findings of Akbey et al.7. Due to the distribution of MIMOSA’s rB1, µMTRRex for values of rB1<0.5 and rB1>1.0 does not influence the overall reproducibility as these rB1 values occur in less than 1% of all pixels in both, WM and GM. In all tissues the reproducibility of the µMTRRex for the different rB1 values is comparable for values of NCorr=2 and 3. For values of 0.75<rB1<0.95, µMTRRex(rNOE) shows a good reproducibility also for Ncorr=0. This is in agreement with our previous findings6. A bias towards higher values of µMTRRex(rNOE) can be observed in images corrected with NCorr=1. The small differences of µMTRRex between the different rB1 values could be explained by the anatomical structure as rB1 changes with the location. Correction with Ncorr=1 requires just half or one third of the required acquisition time in comparison to methods with Ncorr=2 or 3 respectively. An increase of the tSNR of CEST acquisitions should hence follow as less motion and potential B0 drifts influence the final result.
Conclusion
A combination of MIMOSA and B1+ correction with different number of acquisitions used for the correction was implemented and analyzed. Due to MIMOSA’s high homogeneity of the saturation, the B1+ correction methods show good repeatability and reproducibility in the whole brain. Because B1+ correction with Ncorr=2 and 3 proves to be comparable in manner of repeatability to the correction with Ncorr=1 we can advise using MIMOSA with Ncorr=1 saving half the measurement time.Acknowledgements
The financial support of the FAU Emerging Fields Initiative (MIRACLE, support to A.L., K.T. and A.M.N.) is gratefully acknowledged.References
1. Khlebnikov, V., et al., On the transmit field inhomogeneity correction of relaxation-compensated amide and NOE CEST effects at 7 T. NMR Biomed, 2017. 30(5): p. e3687.
2. Windschuh, J., et al., Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed, 2015. 28(5): p. 529-37.
3. Schuenke, P., et al., Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn Reson Med, 2016. 77(2): p. 571-580.
4. Tse, D.H., et al., B1+ inhomogeneity mitigation in CEST using parallel transmission. Magn Reson Med, 2017. 78(6): p. 2216-2225.
5. Liebert, A., et al., Multiple interleaved mode saturation (MIMOSA) for B1 (+) inhomogeneity mitigation in chemical exchange saturation transfer. Magn Reson Med, 2019.
6. Liebert, A., et al. Homogenous Excitation in Whole Brain CEST: Combination of Snapshot CEST and Multiple Interleaved Mode Saturation. in 27th Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine (ISMRM 2019). 2019.
7. Akbey, S., et al., Whole-brain snapshot CEST imaging at 7 T using 3D-EPI. Magn Reson Med, 2019.
8. Schmitt, B., et al., Optimization of pulse train presaturation for CEST imaging in clinical scanners. Magn Reson Med, 2011. 65(6): p. 1620-9.
9. Zaiss, M., P. Ehses, and K. Scheffler, Snapshot-CEST: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed, 2018. 31(4): p. e3879.
10. Fautz, H., et al. B1 mapping of coil arrays for parallel transmission. in Proceedings of the 16th Annual Meeting of ISMRM, Toronto, Canada 2008.
11. Zu, Z., et al., Optimizing pulsed-chemical exchange saturation transfer imaging sequences. Magn Reson Med, 2011. 66(4): p. 1100-8.
12. Penny, W.D., et al., Statistical parametric mapping: the analysis of functional brain images. 2011: Elsevier.
13. Zhang, Y., M. Brady, and S. Smith, Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging, 2001. 20(1): p. 45-57.
Figures