3085
Hyperpolarization and 1H-based detection of [15N]carnitine, a new long-lived metabolic imaging probe
Cornelius von Morze1, James D Quirk1, John A Engelbach1, Galen D Reed2, Albert P Chen2, Craig R Malloy3, Joel R Garbow1, and Matthew E Merritt4
1Department of Radiology, Washington University, St Louis, MO, United States, 2GE Healthcare, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern, Dallas, TX, United States, 4Department of Biochemistry and Molecular Cell Biology, University of Florida, Gainesville, Gainesville, FL, United States
1Department of Radiology, Washington University, St Louis, MO, United States, 2GE Healthcare, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern, Dallas, TX, United States, 4Department of Biochemistry and Molecular Cell Biology, University of Florida, Gainesville, Gainesville, FL, United States
Synopsis
13C hyperpolarization has opened the door for real-time MR metabolic imaging but remains limited by T1's of approximately one minute or less. 15N offers potentially much longer T1's, but suffers from poor intrinsic sensitivity and small chemical-shift separation. We have hyperpolarized L-[15N]carnitine-d9, a novel metabolic imaging probe, and found ultra-long T1's of 210s (in H2O) / 160s (in vivo). We also demonstrate successful 15N->1H hyperpolarization transfer for enhanced sensitivity of detection as well as larger chemical-shift separation among metabolites. The long signal lifetime and excellent safety profile of [15N]carnitine suggest great potential for metabolic imaging investigations and future clinical translation.
Purpose
Hyperpolarization has enabled imaging of real-time metabolic activity, yet is limited by the rapid decay of magnetization via T1 relaxation. While 13C nuclei are generally limited to T1’s of about one minute or less, protected 15N positions are known to exhibit T1’s as long as several minutes1. However, 15N detection is intrinsically insensitive and longer-lived sites typically exhibit only tiny chemical-shift changes upon metabolic conversion, limiting the applicability of 15N for in vivo metabolic investigations. Thus, a potentially ideal approach is storage of hyperpolarization at 15N followed by polarization transfer to J-coupled protons for detection with enhanced sensitivity and chemical-shift separation among metabolites2. Carnitine is a quaternary ammonium compound with key biochemical roles as a buffer for excess acetyl units as well as transport of long-chain fatty acids into mitochondria via CPT1. The purpose of this study was to investigate hyperpolarization and optimized 1H-based detection of HP L-[15N]carnitine-d9 (Fig. 1), a new metabolic imaging probe whose ultra-long T1 (210s in H2O / 160s in vivo) and excellent safety profile are ideally suited for in vivo studies and eventual clinical translation.Methods
DNP: Selective trimethyl deuteration provided added protection from T1 relaxation by 1H-15N dipolar coupling, while preserving transfer targets in the rest of the molecule, in particular the methine resonance with 3JNH~3.6Hz. L-[15N]carnitine-d9 (Cambridge Isotopes) was dissolved in water/DMSO to 4M, with trityl radical OX063. Each sample was loaded into the GE SPINLab hyperpolarizer (5T/0.8K), irradiated @ 140GHz and rapidly dissolved in superheated H2O, giving a 16mM aqueous solution of HP [15N]carnitine. HP carnitine solution was transferred to a Varian/Agilent 4.7T small animal scanner located one floor below the polarizer. MR Hardware: During sample transfer (~30s), the ambient magnetic field around the sample was controlled at ~50 Gauss using a home-built, hand-held solenoidal electromagnet (Fig. 2A). A home-built 5cm dual-tuned 15N/1H (20.2MHz/199.3MHz) square transceiver RF surface coil was also constructed for this study (Fig. 2B). MR experiments: 15N pulses were calibrated using a 5mL 10M [15N2]urea vial phantom. Aqueous and in vivo 15N T1’s were estimated by fitting dynamic HP 15N data obtained by repetitive low flip angle, hard-pulse excitation (5 every 30s). For the in vivo study, a normal rat was anesthetized and injected with 2.5mL of the 16mM HP carnitine solution via tail vein, with RF coil placed anteriorly over the rat liver. For developing optimized 1H-based detection, a vendor-supplied refocused INEPT sequence was modified with added 10:1 coherence selection gradients for specifically detecting polarization transferred from 15N to 1H (Fig. 3), and tested on a syringe containing 3mL of 16mM HP carnitine. In anticipation of 1H-detected in vivo metabolic studies, the 1H chemical shift between carnitine and acetylcarnitine at the methine position was measured by 1H MR of a reference solution and compared against spectral databases.Results and Discussion
Estimated T1’s determined from fitting dynamic low flip angle HP [15N]carnitine data (Fig. 4A) acquired at 4.7T were 210s (in H2O) and 160s (in vivo). The DNP enhancement factor was estimated to be ~57,000 at 4.7T, based on comparison with [15N2]urea phantom data, corresponding to a polarization level of 11%. Remarkably, the [15N]carnitine coherence was directly detectable in rat liver for over 5 minutes (Fig. 4B), despite the relatively low dosage (~7mg carnitine injected) and the intrinsically low sensitivity of 15N. Injection was tolerated well, consistent with the excellent safety profile of L-carnitine. Applying the new INEPT sequence with gradient-based coherence selection eliminating water and any other 1H resonances, 15N hyperpolarization was successfully transferred to protons in carnitine (Fig. 5), including some inadvertent transfer to both sets of methylene protons. The 1H chemical-shift difference between carnitine and acetylcarnitine in an aqueous reference solution was measured to be 1.0ppm (which can readily be resolved in proton spectroscopic imaging), in good agreement with spectral databases. Large quantities of acylcarnitines, especially acetylcarnitine, are known to be formed within minutes after intravenous injection of carnitine at similar dose levels used for hyperpolarized MRI3, indicating strong potential for metabolic studies using this new probe. In comparison with the gold standard MR metabolic imaging probe [1-13C]pyruvate, the T1 of [15N]carnitine is 3x longer in H2O and ~4-5x longer in vivo. A limitation of this study that will be addressed soon was the use of surface coils, as uniform B1 transmission is highly desirable for maximally efficient polarization transfer experiments.Conclusion
We have achieved hyperpolarization and 15N->1H hyperpolarization transfer for improved detection of HP L-[15N]carnitine-d9, a new MR metabolic imaging probe with ultra-long T1.Acknowledgements
The authors are grateful to JJH Ackerman for his valued consultation and enthusiastic encouragement, as well as the support of the Mallinckrodt Institute of Radiology. Grants: NIH S10OD023580, K01DK099451, R01DK115987.References
- Nonaka H, Hirano M, Imakura Y, Takakusagi Y, Ichikawa K, Sando S. Design of a 15N Molecular Unit to Achieve Long Retention of Hyperpolarized Spin State. Scientific Reports. 2017.
- Sarkar R, Comment A, Vasos PR, Jannin S, Gruetter R, Bodenhausen G, Hall H, Kirik D, Denisov VP. Proton NMR of 15N-Choline Metabolites Enhanced by Dynamic Nuclear Polarization. JACS. 2009.
- Brass EP and Hoppel CL. Relationship between acid-soluble carnitine and coenzyme A pools in vivo. Biochem J. 1980.
Figures
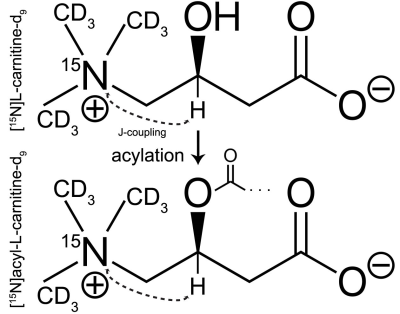
Fig. 1. Structure and isotopic labelling of [15N]carnitine used in this study, showing metabolic acylation reaction occurring in vivo. The methine resonance is the target for hyperpolarization transfer for 1H-based detection, with sufficient coupling (3JNH~3.6Hz) and significant 1H chemical-shift difference for resolving metabolic conversion to acetylcarnitine (1.0ppm).
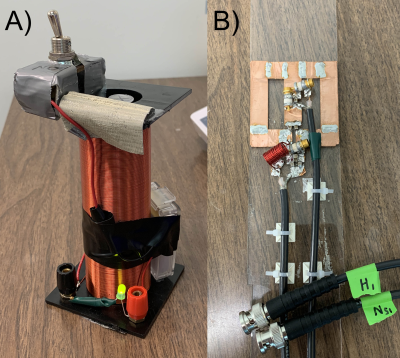
Fig. 2. Home-built MR hardware for HP 15N studies, A) 50 Gauss electromagnet for HP sample transfer, B) 5cm dual-tuned 15N/1H RF transceiver surface coil.
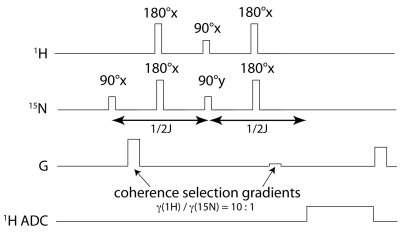
Fig. 3. Gradient-enhanced refocused INEPT pulse sequence for selective 1H-based detection of HP 15N magnetization, with 1/2J=139ms for the case of transfer to methine proton in [15N]carnitine.
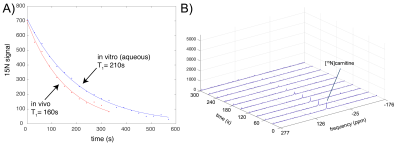
Fig. 4. Estimated T1 decay curves (A) for HP [15N]carnitine in H2O (blue) and in rat liver in vivo (red). Data sets are scaled to the same value at t=0. In (B), in vivo dynamic HP 15N spectra of [15N]carnitine in rat liver, showing detection of the [15N]carnitine peak (~7mg carnitine injected) for 5 minutes after injection.
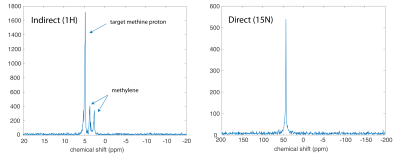
Fig. 5. Comparison of indirect 1H and direct 15N (5°) detection of HP [15N]carnitine solution.