3084
Assessment of Passive Tumor Targeting of a Novel PFCE-Loaded Nanoemulsion via 19F-MRI1Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 2Morgridge Institute for Research, Madison, WI, United States, 3Pharmacy, University of Wisconsin, Madison, Madison, WI, United States, 4Chemistry, University of Wisconsin, Madison, Madison, WI, United States, 5Biomedical Engineering, University of Wisconsin, Madison, Madison, WI, United States, 6Radiology, University of Wisconsin, Madison, Madison, WI, United States
Synopsis
Fluorine-19 MRI is a method that can utilize biocompatible perfluorocarbons to detect cancer. Implementation of a novel nanoemulsion has allowed for improved particle stability and PFC loading. Tumor-bearing mice were injected with perfluoro-[15-crown-5]-ether (PFCE) loaded nanoemulsion (NE) to explore the passive tumor targeting across 14 days. 19F MRI and subsequent ROI-image analysis suggested that there was a preferential retention of NE particles due to the enhanced permeability and retention (EPR) effect. This work demonstrates repeatable 19F imaging of a novel nanoemulsion that passively targets a fast growing tumor with improved stability and PFCE loading.
Purpose
Fluorine-19 (19F) MRI is an alternative method that can utilize certain stable and biologically compatible compounds known as perfluorocarbons as MRI contrast agents for cancer detection1. A particularly favorable contrast agent, known as perfluoro-[15-crown-5]-ether (PFCE), is very stable and has 20 magnetically-equivalent fluorines that create a single strong peak. To create an injectable form, this agent is encapsulated in polymeric nanoemulsions (NE) of a particular size (<500 nm, according to USP <729> for safe intravenous delivery)2. The purpose of this study was to employ a nanoemulsion prepared with a novel semifluorinated polymer, M2F8H18, with improved PFCE loading and particle stability to explore its improved tolerance and passive tumor targeting in 4T1-Luc tumor-bearing mice.Methods
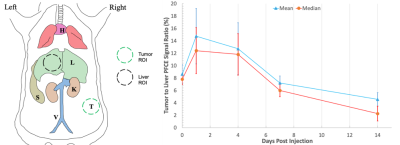
Leveraging the enhanced permeability and retention (EPR) effect of the fast growing solid tumor, tumor-bearing mice, injected with PFCE NE (n = 5), were imaged over the course of 14 days where the ratio of PFCE NE accumulation in tumor to liver was quantified via ROI anaylsis (Figure 2).The novel PFCE NE was prepared using M2F8H18 polymer loaded with 35% (v/v) PFCE as described in Barres et al.3 and particle size was determined via Dynamic Light Scattering (DLS). 4T1-Luc cells, a murine breast carcinoma cell line, were subcutaneously inoculated on the right flank of the female BALB/c mice. After tumors reached 100 mm3, the novel PFCE NE (1.07 M PFCE, 200 µL) was intravenously administered though the tail vein (n = 5). Mice were imaged prior to injection and at 6 hours, 1, 4, 7, and 14 days post injection via 1H and 19F MRI. Mice were induced via 3% isoflurane (ISO) and placed supine into a homebuilt 1H/19F quadrature coil. During imaging sessions, mice were maintained at 37 °C and 1.5% isoflurane.
Anatomic images were acquired using a T2-weighted fast spin echo (FSE) with 0.28 x 0.28 x 2.0 mm3 spatial resolution, 72 x 36 mm2 FOV, 3000/20 ms TR/TE, and 3 averages for a scan time of 1 min 12 sec. 19F images were acquired using a FSE with a 1.1 x 1.1 x 2.0 mm3 spatial resolution, 72 x 36 mm2 FOV, 1250/20 ms TR/TE, echo train length of 8, 128 averages, scan time of 10 min 40 sec, and a saturation pulse at +2150 Hz to avoid any isoflurane contamination. All images were reconstructed and processed using MATLAB 2015b (MathWorks, Natick, MA).
19F images were scaled in arbitrary units of SNR by dividing pixel values by the standard deviation of the background signal and correct for the Rician noise distribution. These images were overlaid with 1H images to provide anatomic context. To quantify passive targeting in the tumor, ROIs were hand drawn along the tumor margin in slices with 19F signal and in the left lobe of the liver. Mean and median liver to tumor ratios were taken for each mouse and time point, and the average and standard deviation was calculated to monitor targeting longitudinally.
Results
The prepared PFCE NE provided a high loading of PFCE (35% v/v) with the average diameter of 218 ± 13 nm. The NE was highly stable over the course of 100 days, indicating negligible particle growth in the novel PFCE NE (data not shown). In vivo 19F-MRI provided detailed NE distribution information in all of the immunocompetent mice. NE is clearly visible in the mice at all time points as seen in a representative mouse shown in Figure 1. At 6 hours post-injection, NE is seen primarily in the liver and spleen and a substantial amount in the heart, vasculature, and kidneys. At days 1, 4, and 7, accumulation at the tumor site is clearly visible, while at day 14, signal decreases uniformly in the tumor, liver, and spleen, suggesting the NE clearance from the body. Tumor to liver accumulation results, shown in Figure 2, indicate tumor to liver ratio spikes at days 1 and 4 and then decreases onward as the NE is cleared from the body. This suggests that the NE is being preferentially retained in the tumor due to the well-known EPR effect, i.e. increased vascular permeability, in fast growing tumors4.Conclusion
In vivo images were acquired for 5 tumor-bearing mice across 14 days. All mice appeared to tolerate the large dose of PFCE NE, as no adverse effects were observed. This study demonstrates repeatable 19F imaging of a novel PFCE NE contrast agent that passively targets the tumor site with improved stability and overall PFCE loading, compared to a typical lipid nanoemulsion. Future studies will look to modify the NE formulation to achieve a smaller particle size (<200 nm) such that liver and spleen uptake is reduced5. Furthemore, the in vivo potential of this PFCE NE to be used as a theranostic agent will be explored, where the NE will carry a therapeutic payload as well as the PFCE imaging agentAcknowledgements
This project supported in part by the University of Wisconsin, Departments of Medical Physics, Human Oncology and Radiology, Cancer Pilot Funding from the UW-Carbone Comprehensive Cancer Center CCSG P30CA014520NIH/NCI R01 CA21546, and by an AOF/SciMed GRS Fellowship to Lawrence Lechuga by the Office of Graduate Education, UW-Madison.References
1. Ratner, A. V. et al. Detection of tumors with 19F magnetic resonance imaging. Invest. Radiol. (1988). doi:10.1097/00004424-198805000-00006
2. Fanun, M. Colloids in Drug Delivery. 150, (CRC Press, 2010).
3. Barres, A. R., Wimmer, M. R. & Mecozzi, S. Multicompartment Theranostic Nanoemulsions Stabilized by a Triphilic Semifluorinated Block Copolymer. Mol. Pharm. 14, 3916–3926 (2017).
4. Golombek, S. K. et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 130, 17–38 (2018).
5. Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Figures