3080
PET image quality improvement for PET/MR with low-attenuating AIR Technology coils1GE Healthcare, Waukesha, WI, United States, 2University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Floating surface coils, such as the anterior array coil, are ignored in PET attenuation correction for PET/MR because their position is difficult to determine. This leads to quantitative bias in reconstructed PET images. This study investigates the potential to improve quantitative accuracy in PET when using a new lightweight "AIR Technology" anterior array coil, as compared to the traditional anterior array coil. Metrics of quantification accuracy and error variation were explored with a phantom and clinical study.
Introduction
Simultaneous PET/MR presents a series of challenges for quantitatively accurate and consistent PET imaging. One such challenge is the presence of floating MR surface coils for body imaging, most notably a (semi-)flexible anterior array (AA) coil. Because the position of the coil on the patient is difficult to determine, AA coils are often excluded from attenuation correction processing for PET imaging, leading to unknown quantitative bias. Recently, a light-weight coil technology has been developed called AIR Coil Technology. These coils are reduced in weight by approximately 60%, which translates to less photon attenuation and is expected to reduce the impact of the RF coil on the PET image data. The purpose of this study is to evaluate the potential PET image quality improvements when using the AIR AA coil as compared to the traditional AA coil.Methods
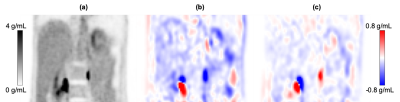
Phantom study: A Ge-68 phantom was scanned on a PET/CT system (Discovery MI, GE Healthcare) with multiple coil configurations: (a) no AA; (b) traditional AA; (c) AIR AA. For each configuration, AC was performed without the AA, mimicking the processing on a clinical PET/MR scan. Images were reconstructed with and without time-of- flight (TOF) PET reconstruction. For each slice, 25 ROIs with 25 mm diameter were placed throughout the slice. The mean of the ROIs was used for quantitative analysis, and the coefficient of variation (CV) of the ROI values was used for homogeneity analysis.Injected volunteer study: Following an F-18-FDG PET/CT clinical scan, 2-min PET frames were acquired in the same position over the liver without moving the patient. Four scenarios were scanned on PET/CT: (a) no coil; (b) AIR AA; (c) no coil (repeated); (d) traditional AA. The original CT scan (without the AA coils being present) was used for AC for all reconstructions, mimicking the scenario of simultaneous PET/MR scan. TOF PET reconstruction was performed. A large spherical VOI was placed identically near the center of the liver for each reconstructed volume. The VOI “gold standard” for each coil configuration was calculated, using a line-fit (in time) of the no-coil VOI means as the ground truth (to account for potential biological change).
Results
Phantom study: The traditional AA showed mean quantitation error of -9.0% & -8.6% (TOF & non-TOF, reported in this manner for all measurements), reduced with the AIR AA to -4.3% & -4.0%. The coefficient of variation with no coil was 2.0% & 1.5%; with the traditional AA the CV was 4.3% & 6.2%; with the AIR AA the CV was improved to 2.1% and 2.7%.Volunteer study: The liver VOI demonstrated an error of -9.9% & -9.0% with use of the traditional AA; with the AIR AA error was reduced to -3.6% & -3.6%.
Conclusion
Phantom and volunteer studies demonstrated that AIR Technology coils reduce mean quantitative bias by over 50%. Additionally, the spatial variation of the error was reduced, which would improve reliability of quantitative analysis for longitudinal studies. The phantom studies demonstrated similar mean quantitation error between TOF and non-TOF reconstruction, but the TOF reconstructions demonstrated superior homogeneity as compared to non-TOF when coils were omitted from the AC map. Given that contemporary MR-based attenuation correction (MRAC) algorithms have demonstrated bias in most tissue regions on the order of 10% of less, it is important to consider how other factors such as RF coils effect quantitative results in PET, as coil-related bias can be as large as those due to MRAC. With the use of lightweight AA coils using AIR Coil Technology or similar technologies, quantitative bias can be greatly reduced, improving quantitative PET results while balancing patient comfort and imaging capability.Acknowledgements
The authors acknowledge support from the NIH (R01-EB026708).References
[1] Wollenweber SD, et al. “Characterization of the impact to PET quantification and image quality of an anterior array surface coil for PET/MR imaging.” MAGMA. 27.2 (2014): 149-159.
[2] McGee KP, et al. “Characterization and evaluation of a flexible MRI receive coil array for radiation therapy MR treatment planning using highly decoupled RF circuits.” Phys Med Biol. 2018;63:08NT02.
Figures

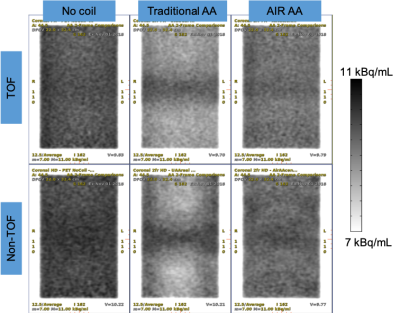
Figure
2: These coronal images demonstrate:
-Reduced
error with AIR AA as compared to traditional AA.
-Better
homogeneity with TOF recon when a coil is unaccounted for in attenuation
correction.
Notes: 12.5 mm thick; image plane above central axis by 45 mm; narrow viewing window.