3079
Evaluation of Cucurbit[6]uril Diffusion Across an Artificial Blood-Brain Barrier1Biology, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Research Institute, Thunder Bay, ON, Canada, 3Chemistry and Material Science, Lakehead University, Thunder Bay, ON, Canada, 4Chemistry, Lakehead University, Thunder Bay, ON, Canada, 5Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
The selective nature of the blood-brain barrier (BBB) is highly regarded when evaluating the permeability of a molecule such as Cucurbit[6]uril (CB6). CB6 has previously demonstrated a strong hyperCEST effect, which makes it a valid candidate for detecting low concentrations of biomarkers of neurodegenerative pathologies. However, CB6 must be permeable to the BBB to use the hyperCEST technique for neurological biomarkers. Therefore, this work evaluates the permeability of CB6 across an artificial BBB based on diffusion. It was found that CB6 can cross the brain sphingomyelin powder (BSM) based artificial BBB.
Introduction
Cucurbit[6]uril (CB6) has been identified as a potential biosensor imaging agent for neurodegenerative pathologies. CB6 is a supramolecular cage molecule that reversibly encapsulates hyperpolarized (HP) 129Xe atoms.1 However, a HP 129Xe biosensor requires a binding component to become functionalized for in vivo studies. Thus, the next step would be to functionalize CB6 with a binding component.2 The hyperpolarized chemical exchange saturation transfer (hyperCEST) technique could be an effective way to detect neurodegenerative pathologies such as Alzheimer’s and Parkinson’s disease at the early stages using CB6 as a biosensor in vitro, as CB6 demonstrates a strong hyperCEST effect. In addition to the strong hyperCEST from CB6, it also must cross the blood-brain barrier (BBB) in order to interact with 129Xe in the brain for imaging 2,3. The human BBB is a semi-permeable membrane that is highly selective. The permeability of the BBB is highly dependent on the size and polarity of the molecule, such that passive diffusion will occur only in molecules that have a small molecular weight and non-polar character.4 This study tested the ability of CB6 to cross an artificial porcine brain sphingomyelin powder (BSM) based BBB, using a method similar to Parallel Artificial Membrane Permeation Assay (PAMPA)5, to determine if it could be used as a valid biosensor for the hyperCEST imaging technique.Methods
An artificial BBB permeability membrane was constructed by coating porcine BSM (Avanti Polar Lipids Inc., Alabaster, AB, USA) onto a polytetrafluoroethylene (PTFE) syringe filter. A 0.5 mg/mL solution of BSM was prepared using deionized distilled water (ddH2O). Four drops of the BSM solution were placed into the surface of a 0.45 µm PTFE syringe filter and sat for 12 hours. A sample was prepared using a 5 mM CB6 (Sigma Aldrich) solution in ddH2O water. This sample was placed onto the BSM coated PTFE filter. A syringe containing the CB6 and ddH2O water solution was secured to the top of the PTFE filter, while the collecting end emptied into a microcentrifuge tube. This system was left for 20 hours to diffuse. The pre and the post diffusion samples were emptied into separate vials and underwent evaporation using a Buchi Rotavapor® R-300 to eliminate the solvent from the tube. The remnants in the tube were dissolved in 500 µL of DMSO-d6 to prepare for 1H NMR. A CB6 reference sample was prepared for 1H NMR, containing 500 µL of DMSO-d6 and CB6 hydrate. The CB6 reference, pre and post-diffusion of CB6 samples were analyzed with 1H NMR (relax delay=1.000 sec, pulse=45.0 degrees, acquisition time=2.049 sec, width=5996.6 Hz, 64 repetitions). 70% ethanol was the positive control and was analyzed pre and post-diffusion using 13C NMR (relax delay=1.000 sec, pulse=45.0 degrees, acquisition time=1.300 sec, width=30154.5 Hz, 256 repetitions). 50 µL of ddH2O was added to the NMR tube to act as a reference.Results and Discussion
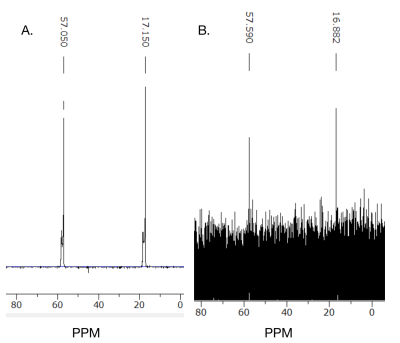
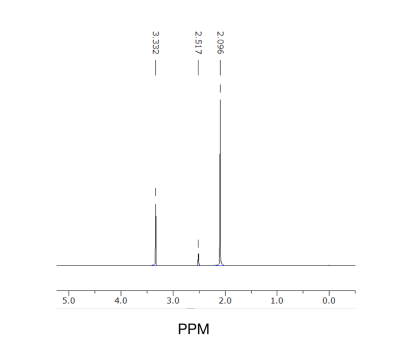
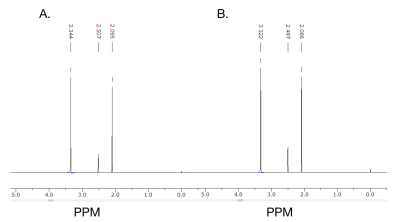
Based on the 13C NMR spectra, the positive control ethanol penetrated the artificial BSM based BBB. Ethanol was identified on both of the 13C NMR spectra from the pre and post-diffusion samples obtained (Figure 1). However, the amount of ethanol that was detected in the post membrane (Figure 1B) was lower than the pre (Figure 1A). Therefore, this diffusion method across the artificial BBB was repeated using CB6 to determine its permeability. Pure CB6 was dissolved in d6-DMSO as a reference, which was used to compare the pre-diffusion 5 mM CB6 (Figure 2). The pre and post-diffusion across the artificial BSM based BBB of the CB6 samples were analyzed using 1H NMR, in which CB6 was identified on both spectra (Figure 3). The CB6 identified in the post-diffusion spectra had a very minimal concentration (Figure 3B). Therefore, based on the results that show the permeability of CB6 in vitro, there is potential for CB6 to cross the BBB for in vivo studies. Thus, CB6 could potentially be functionalized to use as a biosensor to precisely detect markers of early brain disease such as Alzheimer’s and Parkinson’s disease in vivo.2 To increase the concentration of CB6 crossing the artificial BBB, a practical method would be the addition of lipophilic groups to CB6, which would help encourage passive diffusion of the molecule.6Conclusion
We demonstrated that CB6 can cross an artificial BSM based BBB in an in vitro model. Therefore, given the capability of CB6 to cross the artificial BSM based BBB, we anticipate that CB6 should have a similar affinity for crossing the human BBB in vivo. Thus, there is potential for CB6 to be developed as a biosensor for various neurodegenerative pathologies such as Alzheimer’s and Parkinson’s disease, at early stages.Acknowledgements
This study was funded in part by the Natural Science and Engineering Research Council (NSERC) of Canada Discovery grant. C.J.N. was supported by an NSERC Undergraduate Student Research Award (USRA). F.T.H. was supported by the BrightFocus Foundation and the Canadian Institutes for Health Research. V.G. was supported by an Ontario Trillium Scholarship. We would like to thank Michael Sorokopud and Vincent Trinh for their assistance.References
1. Hane F, Li T, Smylie P, et al. In Vivo Detection of Cucurbit[6]Uril, a Hyperpolarized Xenon Contrast Agent for a Xenon Magnetic Resonance Imaging Biosensor. Sci Rep. 2017; 7, 41027.
2. Spence M, Rubin S, Dimitrov I, et al. Functionalized Xenon as a Biosensor. Proc Natl Acad Sci USA. 2001;98(19):10654–10657.
3. Schröder L, Lowery T, Hilty C, et al. Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science. 2006;314(5798):446–449.
4. Ballabh P, Braun A, Nedergaard M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiology of Disease. 2004;16(1):1–13.
5. Kansy M, Senner F, Gubernator K. Physicochemical High Throughput Screening: Parallel Artificial Membrane Permeation Assay in the Description of Passive Absorption Processes. J. Med. Chem. 1998;41(7):1007–1010.
6. Fong C. Permeability of the blood-brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. JMB. 2015;248(4):651-669.
Figures