3078
In-Vitro HyperCEST detection of decacationic pillar-5-arene1Biology, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Research Institute, Thunder Bay, ON, Canada, 3Chemistry and Material Science, Lakehead University, Thunder Bay, ON, Canada, 4Chemistry, University of Rhode Island, Kingston, RI, United States, 5Chemistry, Lakehead University, Thunder Bay, ON, Canada, 6Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Hyperpolarized (HP) 129Xe molecular imaging technology has been working towards the detection of biochemically inactive supramolecular cage-molecules within a living mammalian model. The issue originates from the non-specific natural biodistribution of the biosensor molecules, which makes it difficult to precisely localize them within a living mammalian body using HP 129Xe MRI. We have previously identified cyclodextrin-based pseudorotaxanes and benzene-appended CB6 as conjugatable scaffolds for xenon biosensors; in this work, we introduce a third class of conjugatable scaffolds, with the hyperCEST detection of pillar[5]arene, a potential precursor to a large variety of targeted molecular imaging probes.
Introduction
Hyperpolarized Chemical Exchange Saturation Transfer (hyperCEST) is a potentially powerful tool for molecular imaging1. During the last decade, the hyperCEST effect was demonstrated with a variety of supramolecular cages such as cryptophanes A2, cucurbiturils1,3, and pseudorotaxanes4,5. Among all studied macrocycles, currently, only Cryptophane A was fully functionalized however the functionalization procedure is difficult and produces a low yield of the final biosensor. Recently, the hyperCEST effect was demonstrated from pillar[5]arene6 supramolecular cage. The purpose of this work was to show that the decacationic pillar[5]arene with 10 methylimidazolium groups can be a sufficient host for hyperCEST. It was shown that this molecule can be easily synthesized with a high yield and it potentially can be functionalized for molecular imaging purposes7.Methods
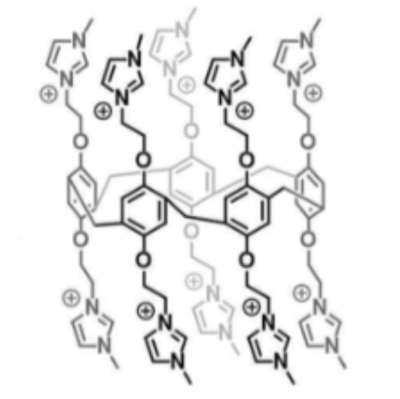
The desired pillar-5-arene was synthesized using the scheme reported by Fernando, et al7. The molecular structure of studied P5A is shown on Fig. 1. Naturally abundant 129Xe gas was polarized to ~50% via the spin-exchange optical pumping (SEOP) technique using a Xemed polarizer (Xemed, Durham, NH, USA). It was then placed into a 1L Tedlar bag which was immediately moved into a pressurized chamber within the bore of a Philips Achieva 3.0T clinical MRI scanner. A ~2.5 ml sample of 10 mM P5A solution in DMSO was drawn into the phantom vessel located inside a custom-built quadrature coil tuned to Xe resonance frequency (35.33 MHz). HP 129Xe was continuously bubbled into solution while hyperCEST spectra were simultaneously acquired using SV MRS technique. The following spectroscopy parameters where used: TR/TE =4s/0.17ms, excitation BW = 1424 Hz, receiving BW = 32 kHz, sampling number = 2048. The 16 sinusoidal pulses of 30 ms duration and 1530 O flip angle were used as a saturation pulse train. The 2 ppm frequency shift was used and the saturation frequency varied from -150 to +30 ppm with respect to the frequency of Xe dissolved in DMSO. All obtained Xe spectra were analyzed using home-built MatLab script in MATLAB R2016b (The Mathworks, Inc, Natick, MA) and the hyperCEST depletion spectrum was plotted. It was exported from MatLab to OriginPro 2016 software (OriginLab Corp., Northampton, MA), The spectrum was denoised using the low-pass parabolic Fast Fourier Transform filter. The pass frequency was equal to 0.063 Hz and the stop frequency was equal to the 0.142 Hz. Denoised spectrum was fitted using two Lorentzian peaks.Results and Discussion
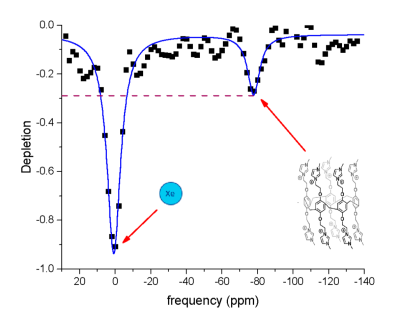
The obtained hyperCEST depletion spectrum, fitted with two Lorentzian peaks is shown in Fig.2. A peak at 0 ppm corresponds to HP 129Xe dissolved in DMSO. Maximum depletion of studied P5A was observed to be 27% at – 78 ppm with respect to the dissolved phase 129Xe signal. This depletion value is close to the depletion reported by Schnurr6 for a different P5A probe indicating that methylimidazole groups do not significantly affect the interaction between the central cavity and dissolved HP Xe. Although P5A hyperCEST depletion tends to be approximately 2.6 times lower compared to results obtained from cucurbit[6]uril8, studied P5A still can be used for molecular imaging purposes. The further improvement of hyperCEST performance of P5A can be done by adjusting parameters of the saturation pulse train such as pulse duration and B 1 magnetic field.Conclusion
We showed the hyperCEST effect of pillar-5-arene with ten cationic methylimidazolium groups. The hyperCEST depletion was similar to the value of other pillar-5-arenes and was equal to 27%. Overall, our study shows that decacationic P5A is a promising candidate for molecular imaging and can be used for further 129Xe biosensor synthesis.Acknowledgements
F.T.H. was supported by the BrightFocus Foundation and the Canadian Institutes for Health Research. Y.S. was supported by an Ontario Graduate Scholarship. V.G. was supported by an Ontario Trillium Scholarship. MSA thanks Dilip Balamore for stimulating discussions.References
1. Hane FT, Li T, Smylie P, et al. In vivo detection of cucurbit[6]uril, a hyperpolarized xenon contrast agent for a xenon magnetic resonance imaging biosensor. Sci Rep. 2017;(3).
2. Bai Y, Hill PA, Dmochowski IJ. Utilizing a water-soluble cryptophane with fast xenon exchange rates for picomolar sensitivity NMR measurements. Anal Chem. 2012;84(22):9935-9941.
3. Wang Y, Dmochowski IJ. Cucurbit[6]uril is an ultrasensitive 129Xe NMR contrast agent. Chem Commun. 2015;51(43):8982-8985.
4. Hane FT, Fernando A, Prete BRJ, et al. Cyclodextrin-Based Pseudorotaxanes: Easily Conjugatable Scaffolds for Synthesizing Hyperpolarized Xenon-129 Magnetic Resonance Imaging Agents. ACS Omega. 2018;3(1).
5. Slack CC, Finbloom JA, Jeong K, et al. Rotaxane probes for protease detection by 129Xe hyperCEST NMR. Chem Commun. 2017;53(6):1076-1079.
6. Schnurr M, Joseph R, Naugolny-Keisar A, et al. High Exchange Rate Complexes of 129 Xe with Water-Soluble Pillar[5]arenes for Adjustable Magnetization Transfer MRI. ChemPhysChem. 2018;20(2):246-251.
7. Fernando A, Mako TL, Levenson AM, et al. A polycationic pillar[5]arene for the binding and removal of organic toxicants from aqueous media. Supramol Chem. 2019;31(8):545-557.
8. Prete BRJ, Robinson D, Fernando A, et al. Benzene-Appended Cucurbit[6]uril as a Potential Biosensor Scaffold for Hyperpolarized 129Xe MRI Molecular Contrast Agents Benzene-Appended Cucurbit[6]uril as a Potential Biosensor Scaaold for Hyperpolarized 129Xe MRI Molecular Contrast Agents. In: Proc. Intl. Soc. Mag. Reson. Med. 26. ; 2018:3034.