3076
Orthotopic Brain Tumor Imaging and Therapy by Microenvironment-Responsive Gold Nanoclusters1Henan Provincial People's Hospital, Zhengzhou, China
Synopsis
Glioblastoma is one of the most lethal cancers with few available therapeutic options. Herein, we report a in situ self-assembled nanoplatform formed by RGD peptide-modified Bisulfite-ZincII-Dipicolylamine-Arg-Gly-Asp (Bis(DPA-Zn)-RGD) and ultrasmall Au-ICG nanoparticles. Taking advantage of RGD-mediated neovascular targeting, the Bis(DPA-Zn)-RGD selectively navigates to the tumor site, and then assembles into large nanoclusters with subsequently administered Au-ICG nanoclusters which can successfully cross the BBB due to ultrasmall particle size (~7 nm). This study demonstrates that such in situ self-assembly metal–organic nanosystems are ideal to overcome the BBB and BBTB, which promise a new way for orthotopic brain tumors imaging and therapy.
Purpose
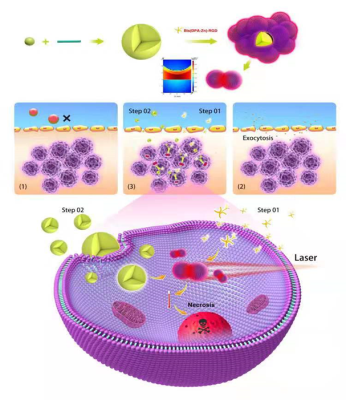
A safer strategy to use small particle size nanocarrier delivery system to pass through the BBB, then nanocarrier responds to stimuli in the local tumor environment so as to be integrated into a larger molecule via self-assembly in the tumor tissue, thereby increasing the retention of the drug at the tumor site for enhanced therapy (Scheme 1).Methods
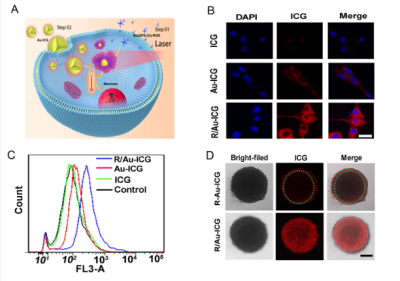
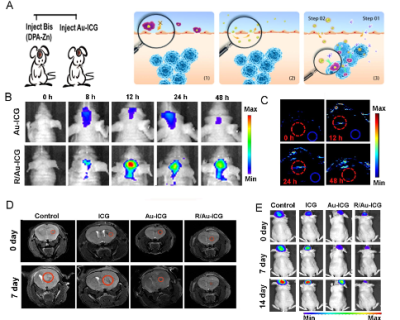
Bis(DPA-Zn) was chemically conjugated with RGD was obtained from professor Gang Liu in Xiamen university [3]. The Au-ICG nanoparticles were synthesized by one step method, due to their particle size, these particles could cross the BBB and further in situ formed Bis(DPA-Zn)-RGD-derived nanoclusters with strong NIR absorption for precise orthotopic glioma imaging. the transmission electron microscopy (TEM) indicated the nanocluster formed by (Bis(DPA-Zn)-RGD) and ultrasmall Au-ICG nanoparticles, the UV-vis absorption of the R-Au-ICG nanoclusters was found to increase from 808 nm to 890 nm, which demonstrated the nanocluster formed, and the enhanced fluorescence imaging(FL), photoacoustic imaging (PA) and photothermal therapy (PTT) properties than control also confirmed the result. In the cells, we studied the using confocal laser scanning microscopy (CLSM), Flow Cytometry, multi-cellular spheroids (MCSs) of U87 cancer cells were incubated to simulate the solid tumor (Figure 2A-D). Before animal testing, male athymic nude mice at 5 week old were anesthetized with isoflurane and fixed on stereotaxic frame. U87 cells expressing firefly luciferase (U87-Fluc) were inoculated to the brain (5 × 103 cells in the striatum: Bregma +1.0 mm, right lateral 2.0 mm, depth 2.5mm). After 7 days, the MRI ,CT combining a bioluminescence imaging was used to monitor the tumor location and growth (Bruker, Germany). Bis(DPA-Zn)-RGD selectively navigate to the tumor site, then with subsequently administered a novel ultrasmall Au-ICG nanoparticles, Furthermore, in the case of Au-ICG triggering by Bis(DPA-Zn)-RGD in the tumor environment produces self-assembly of an R/Au-ICG nanocluster with further enhanced optical properties and targeted thermal ablation of brain tumors (Figure 1a). We studied the fluorescence and photoacoustic imaging of orthotopic tumors and then BLI and MRI were used to monitor tumor growth.Results
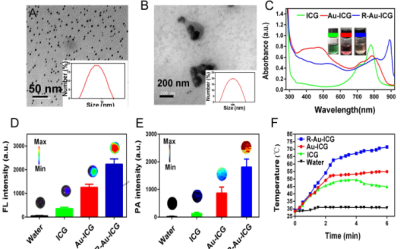
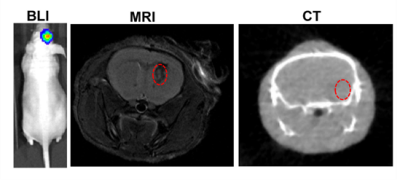
HAuCl4 solution was mixed with the green ICG solution, it turned red in just a few minutes. As shown in Figure 1b (left), When the ICG:HAuCl4 ratio was 1:1, the size of the nanoparticles was ~7 nm (Figure 1A), which provided excellent dispersal and crossing of the BBB, R-Au-ICG nanoclusters are achieved when Bis(DPA-Zn)-RGD interacts with the sulfonate anions of the Au-ICG, TEM revealed that the morphology of nanoclusters were conglobate (i.e. ball-like), with an average diameter of 114 nm (Figure 1B). The UV-vis of the R-Au-ICG nanoclusters was compared to that of ICG and Au-ICG, absorption peak of R-Au-ICG nanoclusters was found to increase from 808 nm to 890 nm (Figure 1C). In addition, the R-Au-ICG nanoclusters exhibited an enhanced fluorescence and PA intensity than control (Figure 1D-E). From the heating curve under the 808 nm laser radiation, we found the R-Au-ICG showed a greater temperature increase and more stable high temperature than ICG and Au-ICG (Figure 1F), possibly because of the greater concentration of ICG in the nanocluster. In U87 cells (Figure 2A), Fluorescence microscopy images and Flow Cytometry showed a higher intensity and longer residence time for R/Au-ICG than for ICG or Au-ICG (Figure 2B-C). By CLSM, for R/Au-ICG red fluorescence extended to the interior of the MCS (∼100 μm) with higher intensity than Au-ICG (Figure 2D), suggesting higher penetration ability of the smaller nanoclusters formed by Bis(DPA-Zn)-RGD and Au-ICG. By applying the MRI, CT and bioluminescence imaging, the formation of intracranial tumors in mice can be also determined (Figure 3). After the injection of Bis (DPA-Zn)-RGD and Au-ICG, a distinct fluorescent signal was detect in the brain area and localized specifically within the U87MG tumor than the other groups, and an obvious PA signal appeared in the brain of tumor mice treated with R/Au-ICG (Figure 4A-C), then mice were exposed to laser irradiation (808 nm, 1.0 W/cm2 for 5 min). MRI revealed that tumor size was much more reduced in the R/Au-ICG group (Figure 4D).Conclusions
In summary, a new type of nanocluster has been synthesized by Bis(DPA-Zn)-RGD instructed self-assembly of small Au-ICG nanoparticles. The Au-ICG nanoparticles were prepared by a one-step approach, crossed the BBB upon systemic administration, and were triggred by Bis(DPA-Zn)-RGD in the tumor environment to self-assemble into nanoclusters, which is suitable for image-guided photo-thermal cancer therapy. R/Au-ICG nanoparticles induced a hyperthermal effect in response to NIR laser irradiation at 808 nm, to effectively suppress brain tumor growth in mice. The high photo-thermal effect of the newly developed Au nanoclusters provides substantial opportunities for broad biomedical applications and further clinical translation.Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFE0103600), National Natural Science Foundation of China (81720108021, 81772009, 81641168, 31470047), Scientific and Technological Research Project of Henan Province (182102310162) and Zhongyuan Thousand Talents Plan Project-- Basic Research Leader Talent (ZYQR201810117)References
1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66: 115-132.
2. Huang H, Liu H, Hsu P, et al. A multitheragnostic nanobubble system to induce blood-brain barrier disruption with magnetically guided focused ultrasound. Adv Mater. 2015; 27: 655-661.
3. Chu C, Ren E, Zhang Y, et al. Zinc(II)-Dipicolylamine coordination nanotheranostics: toward synergistic nanomedicine by combined photo/gene therapy. Angew Chem Int Ed Engl.2019;58:269-272.
Figures