3073
Evaluation of marker-based head motion tracking for prospective correction in MR and retrospective correction in PET for integrated PET/MR1Radiology, University of Wisconsin, Madison, WI, United States, 2Nuclear Medicine Unit, IRCCS Ospedale San Raffaele, Milan, Italy, 3GE Healthcare, Waukesha, WI, United States
Synopsis
Motion during PET acquisition will result in blurring, which may reduce the diagnostic value of the images and cause inaccurate quantitation. Motion during an MR exam results in image replicates and ghosting along the phase encoding direction and other effects due to inconstant phase, rendering images non-diagnostic. In this work, we evaluate the ability of an optical motion tracking system to simultaneously enable prospective correction in MR and retrospective correction in PET of rigid body head motion.
Introduction
Motion during PET acquisition will result in blurring, which may reduce the diagnostic value of the images and cause inaccurate quantitation. Motion during an MR exam results in image replicates and ghosting along the phase encoding direction and other effects due to inconstant phase, rendering images non-diagnostic. Many methods have been proposed in the literature, but no commercial solution exists for arbitrary rigid motion correction in a PET, only basic correction for respiratory and cardiac motion in body. Frame-based image registration techniques have been demonstrated, but have low temporal resolution and can be inaccurate. In MR imaging, external optical systems are available to correct for motion artifacts [1,2]. In this work, we extend the application of an optical MR motion correction system to head FDG PET imaging. Data are acquired such that prospective correction of MR data and retrospective correction of PET list mode data can be accomplished from the same dataset of motion estimates.Methods
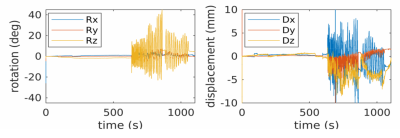
PET data were acquired on a GE SIGNA PET/MR (GE Healthcare, Waukesha, WI, USA). An optical camera (HobbitView Inc., San Jose, CA) was attached to the 8-channel head coil with a 3D printed adapter, and a custom connector located in the scanner bore, allowing fast installation/removal of the camera and straightforward integration with existing patient workflow. The marker consists of an 8.5 x 4 cm curved piece of plastic with a pattern of unique symbols, enabling the camera to identify vertices, and is positioned on the patient’s forehead. Image processing is run in real time on a dedicated Linux server synchronized with the scanner’s clock. Rigid body motion estimates are recorded at 50 frames per second, and used to retrospectively correct list mode data during reconstruction.Seven subjects were scanned with informed consent, in accordance with all local ethics policies, after injection of 18F-FDG and a short uptake duration. A 15-minute time of flight (TOF) PET scan of the head was acquired, along with MR based attenuation correction. The subject was instructed to hold still for 6 mins (static reference data), then gently rock their head from left to right for 6 mins to simulate non-compliant patient motion. Structural MR imaging using 3D BRAVO (TR = 8.2 ms, TE = 3.1 ms, TI = 450 ms, 240x240 matrix, 240 x 240 FOV, 60 slices, 1 mm thk, 1 mm isotropic resolution) was acquired with and without prospective motion correction enabled for both periods of rest and motion during the exam.
TOF-OSEM reconstructions with were performed in MATLAB (R2018b, Mathworks, Natick, MA) using GE Healthcare’s list mode reconstruction software with standard clinical reconstruction settings. Three 5-minute frames were reconstructed for comparison: non-motion reference (static), without motion correction (NoMC), motion correction from camera data (MC).
Results and Conclusions
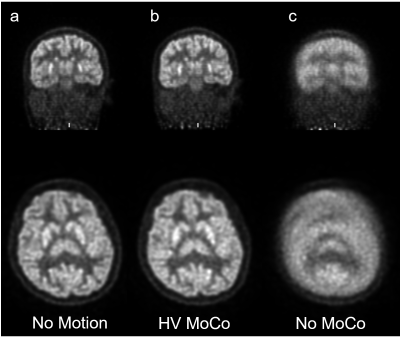
The motion-corrected PET images (MC) exhibited substantial recovery of image fidelity, were of diagnostic quality, and were visually indistinguishable from static reference images, while the uncorrected motion PET images (NoMC) exhibited significant blurring, and were of non-diagnostic quality. The structural MR acquired during the motion portion of the exam, without prospective correction enabled, were also non-diagnostic. MR during the motion period of the exam with prospective correction enabled were of diagnostic quality, although still showed residual artefacts due to motion. Given the large motions subjects were instructed to perform, it is not expected that artefact-free images are recovered, however for smaller, involuntary motions full recovery of image fidelity was observed.Root mean squared error (RMSE) of the PET values in the brain were 3618 Bq/cc for MC and 4592 Bq/cc for NoMC, a 26.1% improvement. Peak signal to noise ratio (PSNR) was 37.5 for MC and 35.4 for NoMC, a 7.7% improvement. Structural similarity index (SSIM) was 0.89 for MC and 0.84 for NoMC, a 5.6% improvement.
In this work, we demonstrate a clinically viable method for prospective correction of structural brain MR imaging and simultaneous retrospective correction of rigid body motion for head PET imaging in a seven subject. This method can enable simultaneous PET/MR imaging of patients with movement disorders (e.g. Parkinson’s disease) where motion is slow and continuous and cannot be broken into discrete frames, eliminate the need to anesthetize pediatric patients for brain scans, and improve the alignment of PET with MR for attenuation correction and anatomical localization. Furthermore, all counts are used in the list mode reconstruction, resulting in high SNR images.
Acknowledgements
The authors acknowledge support from the NIH (R01-EB026708), and financial support from GE Healthcare.References
[1] Maclaren et al. MRM 2012
[2] Maclaren et al. MRM 2017
Figures
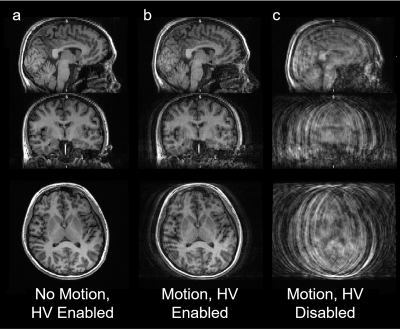
Corresponding structural MR images from the subject presented in Figure 2. Correction of motion during MR occurs prospectively in real time by updating the gradient transform matrix in real time.
Due to the physics of phase and spin histories, prospective correction of large motions is not perfect. However, compared to an image with no correction (c), which is non-diagnostic, the HobbitView system was able to recover diagnostic quality images (b) during large magnitude head rotations. HV correction may also have value in improving quality of MR images in subjects who are compliant (a).