3071
In Vivo Effect of Gadolinium-Based Contrast Agent on PET Quantification for Breast Imaging1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
The effect of gadolinium-based contrast agents (GBCAs) on dynamic PET quantification was investigated. Simultaneous PET/MRI was performed in patients with biopsy confirmed invasive breast cancer. Subjects received an injection of a GBCA as part of standard clinical dynamic contrast enhanced breast MRI exam. SUVmean and SUVmax were calculated for the biopsy-proven breast cancer, aorta, liver, and benign fibroglandular tissue prior to and following GBCA injection. No significant difference in SUVmean or SUVmax was measured suggesting GBCAs are unlikely to affect PET quantification in combined breast PET/MRI.
Motivation
Accurate quantification in PET/MRI relies on proper attenuation correction. PET/MRI studies which use a gadolinium-based contrast agent (GBCA) may result in uncertainties due to increased attenuation by the GBCA which is not accounted for by MR attenuation correction (MRAC). Such errors could be particularly problematic for dynamic PET imaging analysis. While previous work has suggested that GBCAs have no significant effect on the final PET standardized uptake values (SUVs)1,2,3, the work presented here seeks to study this effect using in vivo, dynamic PET/MRI data. In this study, SUVmean and SUVmax pre and post-GBCA injection were compared in patients who received simultaneous 18F-FDG breast PET/MRI exams which included GBCA injection for dynamic contrast enhanced (DCE) MRI.Methods
The study was IRB-approved and HIPAA compliant. Thirteen women ages 33 to 77 years (y) (mean 49 y) with biopsy-confirmed invasive breast cancer consented to undergo simultaneous breast PET/MRI exams. Patients received an IV injection of 18F-FDG (~10 mCi) approximately 85 min (range: 79 to 117 min) prior to simultaneous PET/MRI (SIGNA PET/MR, GE Healthcare, Waukesha, WI). PET acquisition lasted 30 min. Simultaneous MR acquisitions consisted of pre-contrast MRAC, 2D T2-weighted FSE with fat suppression, diffusion weighted imaging scans, and DCE MRI using a 0.1 mmol/kg IV injection of gadobenate dimeglumine (MultiHance, Bracco Diagnostics). The PET images were dynamically reconstructed from list-mode data into 60 frames of 30 seconds each using an OSEM algorithm (3 iterations, 28 subsets, and 4 mm Gaussian filter).A board certified, fellowship-trained breast radiologist drew contours indicating four anatomical regions of interest in each of the 13 subjects. Contours were drawn on the static reconstruction of the full 30-minute PET acquisition. The four contours were (1) the biopsy-proven breast cancer, (2) normal fibroglandular tissue in the contralateral breast, (3) 1 cm diameter spherical region-of-interest (ROI) in the descending aorta, (4) 3 cm diameter spherical volume in the right hepatic lobe.
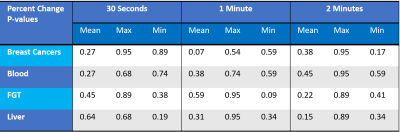
PET frames that began within a 60 second (s) window centered on GBCA injection were used in the analysis (30 s either side of injection). Frames which started before GBCA administration were considered pre-contrast frames and frames which started after GBCA injection were post-contrast frames. The SUVmean and SUVmax of the pre-contrast and post-contrast frames were calculated. A Wilcoxon Rank-Sum Test was used to test for significance in the percent change between pre- and post-contrast frames. Additional analysis was performed using 60 s and 120 s windows on either side of contrast injection.
Results
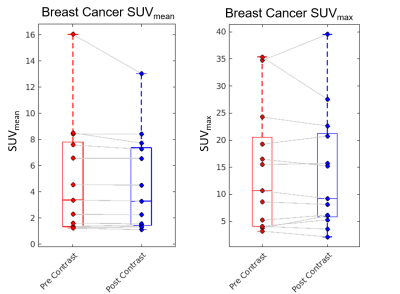
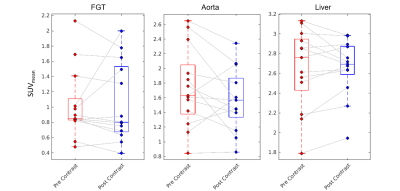
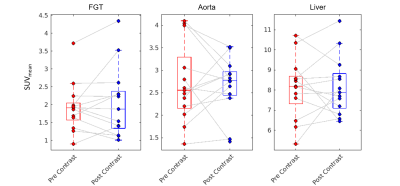
Upon visual inspection of reconstructed images and SUV time-activity curves, no change was perceptible following GBCA administration (Figure 1). In the 30 s timing window, no significant change in SUVmean was measured for the breast cancers (p=0.27, Figure 2), contralateral fibroglandular tissue (p=0.45), descending aorta (p=0.27), or liver (p=0.64, Figure 3). Similar results were obtained for the 60 s and 120 s timing intervals (Table 1). The SUVmax showed no significant change in any region of interest for the 30 s window (breast cancers, p=0.95, Figure 2; FGT, p=0.89; aorta, p=0.68; liver, p=0.68, Figure 4). SUVmax results were similar for the 60 and 120 s timing windows (Table 1).Conclusions
Our results indicate that a standard clinical dose of a GBCA has no significant effect on SUVmean and SUVmax in dynamic PET images. It is possible that a GBCA injection may influence PET signal on time scales smaller than 30 seconds; however, the noise inherent in such short time PET images may be large enough that these effects are undetectable. Further study of GBCA’s effect at short time scales is warranted. This study only looked at a single GBCA: gadobenate dimeglumine. Other GBCAs contain the same ratio of moles of gadolinium to moles of GBCA and would be expected to cause similar levels of attenuation. However, the distribution of gadolinium in a given region may depend on the specific GBCA’s pharmacokinetics, potentially leading to small differences based on GBCA.These findings suggest that SUV corrections due to standard clinical administrations of GBCA are not necessary for dynamic PET images with a 30 second or longer acquisition time. Furthermore, this suggests that GBCAs are unlikely to compromise PET quantitation, including pharmacokinetic modeling of radiotracer uptake, in invasive breast cancer.
Acknowledgements
This project was supported by the Departments of Radiology and Medical Physics, University of Wisconsin. Thanks to Sam Hurley for his assistance in setting up the PET reconstructions.References
1. O’ Doherty J, Schleyer P. An experimental phantom study of the effect of gadolinium-based MR contrast agents on PET attenuation coefficients and PET quantification in PET-MR imaging: application to cardiac studies. EJNMMI Physics 2017; 4:4.
2. Lois C, Bezrukov I, Schmidt H, et al. Effect of MR contrast agents on quantitative accuracy of PET in combined whole-body PET/MR imaging. Eur J Nucl Med Mol Imaging 2012; 39:1756-1766.
3. Lee W, Park J, Kim K, et al. Effects of MR contrast agents on PET quantitation in PET-MRI study. J Nucl Med 2011; 52:53.
Figures