3069
High Resolution Isotropic PET/MR imaging using Anatomical Priors for PET Reconstruction1Radiology, Stanford University, Stanford, CA, United States, 2PET/MR Engineering, GE Healthcare, Waukesha, WI, United States, 3Was with: Nuclear Medicine, IRCCS Ospedale San Raffaele, Milan, Italy, 4Neurology, Stanford University, Stanford, CA, United States, 5Nuclear Medicine, Stanford University, Stanford, CA, United States
Synopsis
The current spatial resolution of PET images is ~4mm for whole body PET/MR. Anatomical MR images with higher resolution and superior SNR, have been used in PET reconstruction to improve the image quality and spatial resolution. However, these methods are generally vulnerable to mismatches between the anatomical image and the true activity distribution. To address this concern, we propose a feature based approach to incorporate anatomical priors in PET image reconstruction, where the location of a voxel or its neighbors doesn't play a direct role and both functional and anatomical images are used to construct the feature space.
Purpose
The resolution of PET images is limited by several physical factors, including positron range, scatter, and the finite size of the detector elements. The current spatial resolution of PET images is ~4mm for whole body PET/MR scanners; however, these scanners are capable of acquiring anatomical MR images with both higher resolution and superior signal to noise ratio (SNR), which can be used in PET reconstruction to improve the image quality [1]. Several segmentation-free methods [2-4] have been proposed to improve the PET image resolution using anatomical information. These methods are generally vulnerable to mismatches between the anatomical image and the true activity distribution. To address this concern, we propose a feature based approach to incorporate anatomical priors in PET image reconstruction, where the location of a voxel or its neighbors does not play a role directly, and instead both functional and anatomical images are used to construct the feature space.Theory
We have used a penalized maximum-likelihood algorithm based on the relative difference prior (RDP), which uses a block sequential regularized expectation maximization (BSREM) optimizer. The RDP applies activity-dependent smoothing to control noise at higher iterations and suppresses image noise in low-activity background regions. This relative difference penalty is calculated based on the difference between each voxel activity and its neighboring voxels as shown in Figure 1(a) [5-6]. We have used the same framework to incorporate the MR anatomical priors into the image reconstruction by applying an additional penalty, which is calculated based on the relative difference between each voxel activity and its neighboring voxels in the feature space as shown in Figure 1(b). The feature space is constructed based on all co-registered multi-parametric functional or anatomical MRI images and an initial PET reconstruction using the conventional OSEM algorithm. By including the conventional PET reconstructed images in the feature space, we can guarantee that the mismatches between the anatomical priors and true activity distribution will not affect the final image.Methods
Two subjects were injected with 8 mCi of FDG and underwent a 60-minute brain scan on a SIGNA PET/MR (GE Healthcare, Waukesha, WI). The study was approved by Stanford's Institutional Review Board and all subjects provided written consent. 3D T1 IR FSPGR, 3D T2 CUBE and 3D T2 FLAIR CUBE images were acquired simultaneously with PET. The PET images were reconstructed with TOF-OSEM with both 2.78 mm and 1.17 mm slice thickness and a transaxial pixel size was 1.17 mm2 (256×256 matrix on a 30 cm FOV). They were also reconstructed using the anatomical priors i.e. 3D T1, 3D T2 and 3D FLAIR images with MR guided TOF-BSREM (MRgTOF-BSREM) using 1.17 mm isotropic resolution.Two other subjects were injected with 5 mCi of RM2 and after 45 min uptake time, they underwent a 20 min prostate exam on SIGNA PET/MR (GE Healthcare, Waukesha). T2 CUBE and DWI were acquired simultaneously with PET. The PET images were reconstructed with TOF-OSEM with isotropic with 2.34×2.34×2.78 mm3 resolution (256×256 matrix on a 60 cm FOV). They were also reconstructed using the anatomical priors i.e. 3D T2 CUBE and DWI images, with MRgTOF-BSREM with 1.17 mm isotropic resolution (512×512 matrix on a 60 cm FOV).Results
Figure 2 shows the comparison between high resolution isotropic images reconstructed by MRgTOF-BSREM and TOF-OSEM as well as the conventional TOF-OSEM reconstruction with 2.78 mm slice thickness. As expected, the high-resolution images reconstructed by TOF-OSEM have lower signal to noise (SNR) ratio and do not show the anatomical boundaries well. In contrast, the MRgTOF-BSREM show an improved SNR and a detailed anatomical boundary compared to TOF-OSEM. Figure 3 shows the fused images of brain PET and MR images in axial and coronal planes. Even though there is a lesion on MR images, it has not impacted the PET images reconstructed by MRgTOF-BSREM due to the construction of the feature space. MRgTOF-BSREM shows higher spatial resolution compared to TOF-OSEM as expected. Figure 4 shows the fused PET and MR prostate images of two subjects in axial, coronal and sagittal planes. The improved spatial resolution of MRgTOF-BSREM compared to TOF-OSEM can be seen in all planes.Conclusion
MRgTOF-BSREM shows better spatial resolution and improved SNR compared to TOF-OSEM in both brain and prostate exam. Because MRgTOF-BSREM uses the feature space and incorporate a conventional PET image reconstruction into the feature space, it is not vulnerable to mismatches between the MR images and true activity distribution.Acknowledgements
References
[1] Bai B, Li Q, and Leahy RM, "MR Guided PET Image Reconstruction", Semin Nucl Med. 2013 Jan ; 43(1): 30-44.
[2] Mehranian A, Belzunce MA, Niccolini F, et al., "PET image reconstruction using multi-parametric anato-functional priors", Phys. Med. Biol. 2017; 62: 5975-6007.
[3] Schramm G, Holler M, Rezaei A, et al., "Evaluation of Parallel Level Sets and Bowsher’s Method as Segmentation-Free Anatomical Priors for Time-of-Flight PET Reconstruction", IEEE Trans Med Imaging. 2018 February ; 37(2): 590-603.
[4] Belzunce MA, Mehranian A, and Reader AJ, "Enhancement of Partial Volume Correction in MR-Guided PET Image Reconstruction by Using MRI Voxel Sizes", IEEE Trans Radiat Plasma Med Sci. 2019 May ; 3(3): 315-326.
[5] Ahn S, Ross SG, Asma E, et al., “Quantitative comparison of OSEM and penalized likelihood image reconstruction using relative difference penalties for clinical PET,” Phys. Med. Biol. 2015; 60: 5733-5751.
[6] Lantos J, Mittra ES, Levin CS, and Iagaru A, "Standard OSEM vs. regularized PET image reconstruction: qualitative and quantitative comparison using phantom data and various clinical radiopharmaceuticals," Am J Nucl Med Mol Imaging. 2018; 8(2): 110-118.
Figures
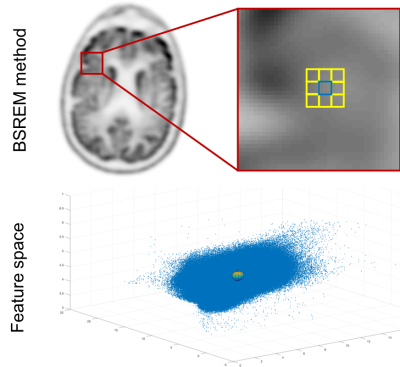
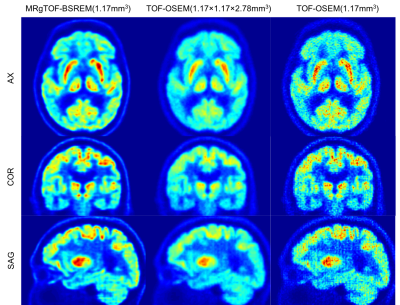
Figure 2: (Right) High resolution isotropic images reconstructed by MRgTOF-BSREM with 1.17 mm slice thickness, (middle) Conventional PET images reconstructed by TOF-OSEM with 2.78 mm slice thickness, and (left) High resolution isotropic images reconstructed by TOF-OSEM with 1.17 mm slice thickness. MRgTOF-BSREM (right) shows a higher resolution and better SNR compared to TOF-OSEM reconstructions (middle and left).
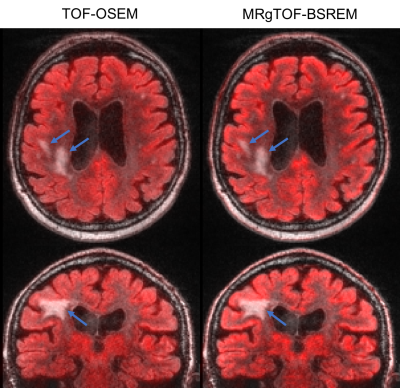
Figure 3: Fused PET and MR brain images using TOF-OSEM with 2.78 mm slice thickness (left) and MRgTOF-BSREM with 1.17 mm slice thickness (right). MRgTOF-BSREM images show a higher spatial resolution and are not impacted by the MR lesion.
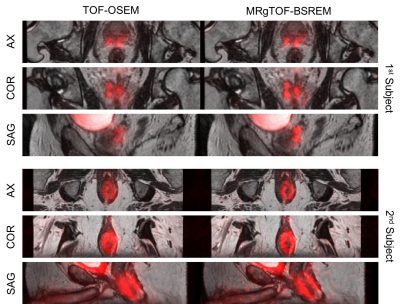
Figure 4: Fused PET and MR prostate images of two subjects using the conventional TOF-OSEM with 2.34×2.34×2.78 mm3 spatial resolution (left) and MRgTOF-BSREM with improved 1.17 mm isotropic spatial resolution (left).