3068
In Vivo Tracking and Quantification of Murine Natural Killer Cells via Fluorine-19 MRI1Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 2Pediatrics, University of Wisconsin, Madison, Madison, WI, United States, 3Carbone Comprehensive Cancer Center, University of Wisconsin, Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin, Madison, Madison, WI, United States, 5Radiology, University of Wisconsin, Madison, Madison, WI, United States
Synopsis
Flourine-19 (19F) MRI has demonstrated the ability to monitor immune cell populations in vivo. Murine green fluorescent protein natural killer (NK) cells were labeled with perfluoropolyether (PFPE-red) and injected intratumorally into lymphoma-bearing mice. In this work, we demonstrate the ability to track and quantify NK cells up to 5 days in vivo, via 19F MRI. In vivo quantification indicated that 88% of the initially detected NK cells remained at the tumor site on day 5. Flow cytometry analysis provided validation that the cellular label remains colocalized within a significant portion of the NK cells at day 5.
Purpose
Our understanding of the cell trafficking behavior and persistence of infused cells in cancer immunotherapy is limited1,2. Fluorine-19 magnetic resonance imaging (19F-MRI) has shown promise for longitudinal monitoring and quantification of immune cell populations in vivo. The purpose of this study was to quantify cell populations of mouse natural killer (NK) cells in-vivo after intratumoral injection longitudinally for up to 5 days with validation of intracellular labeling and persistence using flow cytometry.Methods
Green fluorescent protein (GFP+) NK cells from C57BL/6 mice were labeled for 24-hours with a red-fluorescent perfluoropolyether (PFPE-red; Celsense, Pittsburgh, PA). To determine optimal loading, 19F NMR spectra of lysed-NK cells labeled with various PFPE concentrations were acquired on a 9.4T NMR system (Agilent Technologies, Santa Clara CA) with a TR of 9.0 s, 90-degree flip angle, 14.075 kHz spectral width, and 64 averages. Trifluoroacetic Acid (TFA) was used as a 19F chemical shift and quantification standard. GFP+ NK cells were then labeled with the optimal PFPE concentration, washed, counted, and delivered in equal amounts (1.0⨯106cells/100 μL) intratumorally to three tumor-bearing mice. The mouse tumor model consisted of palpable EL4 T cell lymphoma cells subcutaneously injected on the flank 2 weeks prior. In vivo MRI data was obtained at days 0, 2, and 5 with a 4.7T small animal MRI (Agilent Technologies, Santa Clara CA) using a homemade 1H/19F quadrature coil. Anatomic 1H images were acquired with a T1-weighted gradient echo. 19F images were acquired using a fast spin echo (1000 ms TR, 20.0 ms TE, 1.1⨯1.1⨯2.0 mm3 voxel size, 12 echoes, 14 slices, 360 averages, ~25-minute scan) with an added chemical-saturation pulse to suppress isoflurane frequencies/signals. 1H and 19F images were overlaid using MATLAB 2015B (Mathworks, Natick, MA) to produce composite images. In vivo quantification was normalized to known 19F spin density reference vials that were placed contralateral to the tumor. Apparent NK cell quantification was achieved via region-of-interest (ROI) analysis of 1H/19F images. Images were thresholded at five times the standard deviation of the background noise to obtain true signal and calculate total 19F signal in the tumor and the mean signal in the reference were measured. Quantification and uncertainty calculations were calculated similarly to previously published algorithms3,4. After each imaging day, one mouse was euthanized, and the tumor was excised for analysis via ImageStream flow cytometry (ImageStream MKII, Luminex, Madison, WI) to examine GFP+ and PFPE-red+ populations and validate intracellular PFPE label persistence.Results
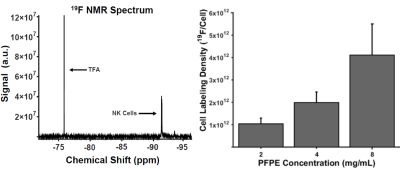
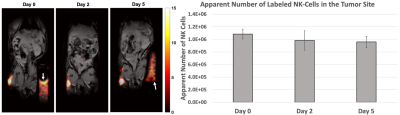
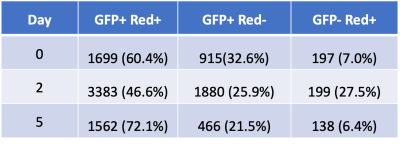
After incubation of NK cells in PFPE-red, NMR analysis indicated 4 mg/mL produced the greatest loading density of 2.0±0.5⨯1012 19F atoms/cell without severely effecting viability and function (Figure 1), an order of magnitude greater labeling density than what was observed in previous work using human-derived NK cells3. In vivo imaging found signal in the tumor on Day 5 (Figure 2), with cell quantification results of 9.6⨯105 cells, or 88% of initially detected apparent NK cells remaining. Flow cytometric analysis confirmed the PFPE-red label remains colocalized within a substantial fraction of GFP+ NK cells at day 5 (Table 1, Column 1), verifying the signal observed by MRI represents a sustained population of 19F labeled NK cells. The presence of unlabeled NK cells (GFP+, Red-) and endogenous phagocytic cells (GFP-, Red+) within the tumor was also observed.Conclusion
In vivo murine NK cells were imaged and quantified within T-cell lymphomas by 19F MRI up to 5 days after intratumoral injection. Necropsies of lymphoma-bearing mice validate the use of PFPE-red as a measure of labeled NK cells in vivo, and show the feasibility of tracking cells by co-localization of 19F conjugated to a distinct fluorophore. Limitations observed included what was likely a small but substantial amount of NK cells releasing 19F upon death and likely being phagocytosed by tumor-associated macrophages (GFP-, Red+), and dilution of the PFPE-label with possible NK cell proliferation or unlabeled cells (GFP+, Red-). Further studies will explore the involvement of macrophages on PFPE uptake, measure long-term (>2-4 weeks) persistence of 19F signal within NK cells, determine a minimum detection threshold, and test detectability of NK cells after intravenous injections.Acknowledgements
This project supported in part by the University of Wisconsin, Departments of Medical Physics, Human Oncology and Radiology, Cancer Pilot Funding from the UW-Carbone Comprehensive Cancer Center CCSG P30CA014520NIH/NCI R01 CA215461, and by an AOF/SciMed GRS Fellowship to Lawrence Lechuga by the Office of Graduate Education, UW-Madison.References
1. Chapelin, F., Capitini, C. M. & Ahrens, E. T. Fluorine-19 MRI for detection and quantification of immune cell therapy for cancer. J. Immunother. Cancer 6, 1–11 (2018).
2. Varani, Auletta, Signore & Galli. State of the Art of Natural Killer Cell Imaging: A Systematic Review. Cancers (Basel). 11, 967 (2019).
3. Bouchlaka, M. N. et al. 19F-MRI for monitoring human NK cells in vivo. Oncoimmunology 5, 1–12 (2016).
4. Srinivas, M., Morel, P. A., Ernst, L. A., Laidlaw, D. H. & Ahrens, E. T. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn. Reson. Med. 58, 725–734 (2007).
Figures