3066
Molecular MRI and synchrotron radiation X-Ray fluorescence of zinc homeostasis in the healthy and malignant mouse prostate1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital/Harvard Medical School, Charlestown, MA, United States, 2Werner Siemens Imaging Center, Tuebingen, Germany, 3Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4McMaster University, Hamilton, ON, Canada, 5Diamond Light Source, Harwell, United Kingdom, 6Division of Surgical Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, United States, 7Department of Chemistry, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Zinc homeostasis is markedly dysregulated in prostate cancer (PCa), and this dysregulation can be detected with glucose-stimulated zinc secretion (GSZS) by MRI. Here we explore the use of GSZS MRI and synchrotron-radiation X-Ray fluorescence to interrogate the effect of dietary zinc on the healthy and malignant mouse prostate. Our results indicate that the lateral lobe of the healthy mouse is the only prostatic structure responsive to a variable zinc diet acting as the zinc “regulator” of the gland, and that in PCa this lobe no longer responds to changes in dietary zinc, highlighting the dysregulation of zinc transporters in PCa.
Introduction
The dysregulation of zinc homeostasis has been used as a biomarker for discriminating PCa from benign prostatic conditions and we discovered that a sudden change in plasma glucose stimulates the secretion of zinc in fasted mice1. This allowed the use of extracellular zinc probes to differentially detect zinc secretion in the healthy and malignant prostate.1-2 Prostate epithelial cells regulate zinc content by transporting and storing the ion via zinc transporters, and in PCa the import transporter ZIP1 is downregulated resulting in prostatic zinc loss3-4. Here, we investigate the effects of a variable zinc diet on the healthy and malignant mouse prostate gland by GSZS MRI and SR-XRFMethods
Synthesis: GdL, a Gd-based zinc probe with high affinity to Zn was synthesized as reported5. Animal model and In vivo MRI: Male 13-wk old C57Bl6 mice and PCa TRAMP mice were fed varying zinc content diets (0.05 ppm Zn, 30 ppm Zn, and 150 ppm Zn, N = 6 each) for 21 days. Mice were imaged at 9.4 T using a Varian/Agilent scanner. Two 3D gradient echo T1-weighted scans were obtained (TE/TR=1.69/3.35ms, Average=4, θ=20°) and mice then received: 0.07mmol/kg GdL (i.v.) plus 2.2 mmol/kg glucose (i.p). Immediately after injections, sequential 3D T1-weighted scans were collected over 10 -15 mins and prostates resected. Synchrotron-Radiation X-Ray Fluorescence (SR-XRF): Prostates were frozen in liq.N2-chilled isopentane, stored at -80oC, sliced into 50 μm-thick sections and mounted on XRF-compatible films. The SR-XRF I18 beam line at the Diamond Light Source was used at 8.2 and 11 keV to collect atomic images of Zn, and Gd. Image analysis: Images were analyzed using ImageJ. Change in contrast to-noise ratio (DCNR) of entire prostate ROIs were quantified and reported as a function of time. The area under the DCNR vs. time curve was calculated and one-way ANOVA analyses were performed using GraphPad Prism. For SR-XRF, the gland was segmented into lateral, dorsal, and ventral lobes and the concentration of Gd and Zn were obtained for each.Results
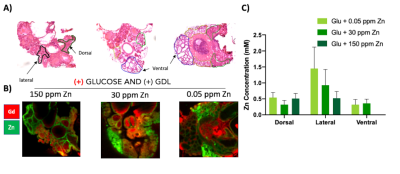
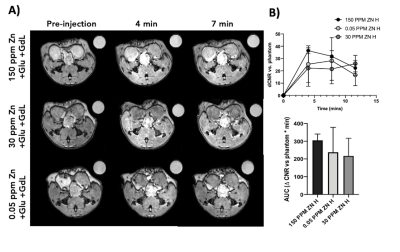
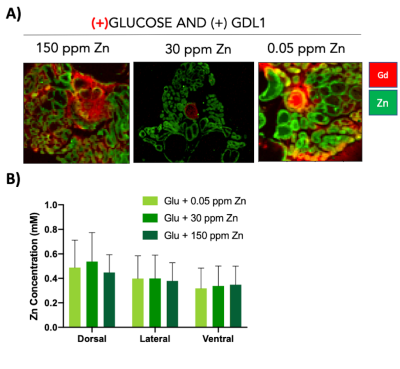
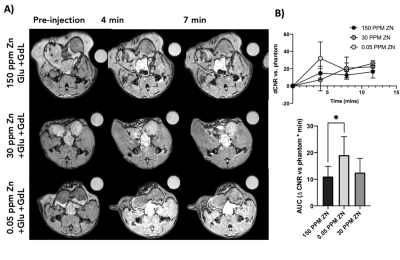
GSZS as detected by MRI is based on formation of a ternary complex between GdL, secreted zinc, and albumin. Here, we correlated zinc content and release in healthy and TRAMP prostate tissues after GSZS of mice fed a zinc deficient (0.05 ppm), sufficient (30 ppm), and excessive (150 ppm) diet. Fig.1A shows H&E-stained prostate sections illustrating the lateral, ventral, and dorsal lobes. And their adjacent SR-XRF-scanned slice showing the distribution of Zn and Gd (Fig.1B). As it was previously shown6 and seen in Fig.1C, under normal zinc dietary conditions the lateral lobe contains the highest levels of zinc: 0.93±0.40mM. This uptake is further exacerbated for the animals under a zinc deficient diet, where the lobe exhibited 1.45 ± 0.60mM. While, a zinc excessive diet did not result in over-accumulation of the metal exhibiting the lowest levels of zinc: 0.52 ± 0.21mM. The lateral lobe is the only prostatic structure that responds to dietary changes by sequestering more or less zinc depending on the bioavailability (Fig.1C). Despite the different levels of zinc content available in the healthy gland from diet, GSZS MRI showed that there were no differences in zinc secretion as detected by GdL (Fig.2A&C). TRAMP mice fed the variable zinc diet showed that the prostate gland did not respond to any dietary demands; Fig 3A&B illustrates the SR-XRF elemental maps of mice fed the high, normal, of low zinc diet and quantitative zinc concentration indicate that there is no difference in lobular zinc content or within dietary levels. However, GSZS MRI results indicate that the animals fed a zinc deficient diet elicited a highest change in signal intensity in the prostate (Fig.4B), and no difference of probe administration was observed (Fig.4B).Discussion and Conclusion
Dietary zinc is absorbed in the jejunum and distributed in the body. In the prostate, it remains mobile and released into seminal fluids but some also redistributed into other compartments in prostate epithelial and stromal cells in response to an increase in plasma glucose. Zinc trafficking in the prostate is facilitated by import and export transporters of the ZIP and ZnT families. It has been hypothesized that the loss of zinc content in PCa cells is initiated by a downregulation of the import transporter ZIP1 located on the cell membrane.3 The dietary changes imposed in this study and their resultant zinc content in the gland is an indirect measurement of the homeostatic status of zinc transporters located in prostate epithelial cells. Exposing healthy cells to deficient levels of bioavailable zinc results in overabsorption into prostatic cells in the lateral lobe likely due to a homeostatic rearrangement of zinc import transporters. Conversely, in PCa the cells are unable to fulfill its homeostatic duties, and when stimulated by glucose they over-secrete intracellular zinc tipping the balance in favor of zinc “wasting”. These results highlight the importance of zinc transporter dysregulation in the onset of PCa and how combining orthogonal imaging technologies such as molecular MRI and synchrotron radiation X-Ray fluorescence enhances our knowledge of metal homeostasis and disease pathogenesis.Acknowledgements
Peter Caravan and his laboratory for insightful feedback.
Funding sources: CPRIT, NIDDK, NHLBI
References
1. Clavijo Jordan, M. V.; Lo, S. T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W. H.; De Leon-Rodriguez, L. M.; Lubag, A. J.; Rofsky, N. M.; Sherry, A. D., Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc Natl Acad Sci U S A 2016, 113 (37), E5464-71.
2. Yuan, Y.; Wei, Z.; Chu, C.; Zhang, J.; Song, X.; Walczak, P.; Bulte, J. W. M., Development of Zinc-Specific iCEST MRI as an Imaging Biomarker for Prostate Cancer. Angew Chem Int Ed Engl 2019, 58 (43), 15512-15517.
3. Franklin, R. B.; Feng, P.; Milon, B.; Desouki, M. M.; Singh, K. K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L. C., hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 2005, 4, 32.
4. Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R. E., The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct Target Ther 2017, 2.
5. Martins, A. F.; Clavijo Jordan, V.; Bochner, F.; Chirayil, S.; Paranawithana, N.; Zhang, S.; Lo, S. T.; Wen, X.; Zhao, P.; Neeman, M.; Sherry, A. D., Imaging Insulin Secretion from Mouse Pancreas by MRI Is Improved by Use of a Zinc-Responsive MRI Sensor with Lower Affinity for Zn(2+) Ions. J Am Chem Soc 2018, 140 (50), 17456-17464.
6. Clavijo Jordan, V.; Al-Ebraheem, A.; Geraki, K.; Dao, E.; Martins, A. F.; Chirayil, S.; Farquharson, M.; Sherry, A. D., Synchrotron Radiation X-ray Fluorescence Elemental Mapping in Healthy versus Malignant Prostate Tissues Provides New Insights into the Glucose-Stimulated Zinc Trafficking in the Prostate As Discovered by MRI. Inorg Chem 2019, 58 (20), 13654-13660.
Figures