3065
Imaging Zinc Dysregulation in Pancreatic Ductal Adenocarcinoma by Secretagogue-Stimulated Zinc Secretion MRI1Division of Surgical Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital/Harvard Medical School, Charlestown, MA, United States, 3Institute for Innovation in Imaging (i3), Massachusetts General Hospital/Harvard Medical School, Charlestown, MA, United States
Synopsis
Zinc homeostasis is markedly dysregulated in pancreatic ductal adenocarcinoma (PDAC), and this dysregulation can be probed by selecting a secretagogue to stimulate the secretion of zinc and functional components from the exocrine pancreas. Here, we introduce the use of caerulein, a surrogate cholecystokinin agonist of pancreatic exocrine secretion, and a Gd-based zinc probe to monitor exocrine function in the healthy and PDAC mouse pancreas by MRI. Our results indicate that zinc dysregulation involves an increase in zinc accumulation in malignant tissue mediated by upregulation of zinc import transporters, and hypersecretory capacity as reported by caerulein-stimulated zinc secretion MRI in vivo.
Introduction
Pancreatic cancer has an overall 5-year survival rate of less than 5%. This poor prognosis is mainly due to the advanced stage at the time of diagnosis.1 Pancreatic surgery for patients who present localized resectable disease is the treatment of choice, but less than 20% of the diagnosed patients qualify for this treatment.2 Therefore, it is crucial to develop technologies for early diagnosis to increase the population of patients who can qualify for curative resections. The exocrine pancreas, composed of acinar and ductal epithelium, secretes 1-2 mg of zinc per day under normal dietary conditions;3 it has been postulated that in pancreatic ductal adenocarcinoma (PDAC) there is a marked dysregulation in zinc homeostasis.4-6 Here, we explore the use of an exogenous secretagogue, caerulein, to stimulate the natural exocrine co-secretion of zymogen enzymes and zinc and a Gd-based zinc probe to report on zinc content and release from the healthy exocrine and PDAC pancreatic tissue in vivo.Methods
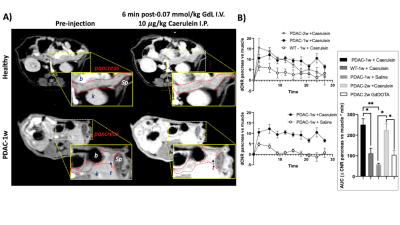
Synthesis: GdL, a Gd-based zinc probe was provided by the Sherry Laboratory.Animal model and In vivo MRI: Male 8-wk old C57Bl6 mice were orthotopically implanted with 105 malignant syngeneic PDAC Hy15549 cells into the pancreas and allowed to proliferate for 1 week (PDAC-1w, N = 6), and 2 weeks (PDAC-2wk, N = 4) as previously reported.8 And healthy male 8-wk old C57BL6 mice as controls (WT, N=5). Mice were fasted overnight and imaged with a 4.7 T Bruker scanner. Two 2D T1-weighted gradient echo scans were obtained (TE/TR = 2.9/125 ms, Avg = 4) and mice then received either 1) 0.07mmol/kg GdL i.v. plus 10 mg/kg caerulein i.p., 2) 0.07mmol/kg GdL i.v. plus Saline i.p., or 0.01 mmol/kg Gd-DOTA i.v. Immediately following injections, serial 2D T1-weighted scans were obtained for 25 minutes.
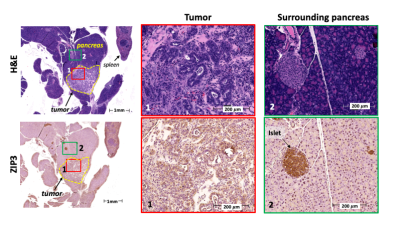
Ex vivo immunohistochemistry: The pancreas was resected, formalin fixed and paraffin embedded for sectioning. Sections were stained with H&E, and a human antibody was used to stain against ZIP3. Image analysis: Images were analyzed using ImageJ. The pancreas tail was located by identifying the spleen, duodenum, and the stomach as anatomical references. Change in contrast to-noise ratio (DCNR) of entire pancreas tail was quantified and reported as a function of time. The area under the DCNR vs. time curve was calculated and one-way ANOVA analyses were performed using GraphPad Prism.
Results
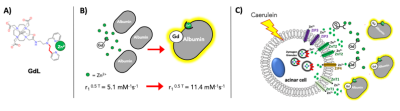
The mechanism of action for secretagogue-stimulated zinc secretion (SSZS) MRI of the exocrine pancreas relies on the identification of the appropriate stimulant (secretagogue) to evoke zinc release from intracellular stores in acinar and ductal cells. By using a Gd-based zinc probe that forms a ternary complex with zinc and serum albumin, thereby promoting an increase in relaxivity (Fig.1A-B), it is possible to detect the stimulated secretion from the exocrine pancreas by monitoring MR signal changes in the tissue as a result of the secretagogue (Fig.1C). We tested this theory by stimulating the secretion of zinc by a caerulein injection in fasted healthy animals where we observed only small enhancement of the pancreas (Fig.2A,top). However, both groups of animals carrying orthotopically implanted PDAC cells (1-wk and 2-wks) responded in a hyper-secretory fashion. Fig.2A, bottom shows a mouse pre-and post-administration of GdL i.v. and caerulein i.p and it is evident that the pancreas and the tumor (1-week old tumor) exhibit significant enhancement compared to healthy controls. Similarly, animals with tumors grown for 2-weeks exhibited similar hyperintensities. Negative controls where PDAC animals did not receive caerulein, and instead received saline, did not show significant pancreatic or tumor enhancement. Fig.2B shows the effect of caerulein in 1-week PDAC animals (bottom), and the increase in overall pancreatic enhancement only found in PDAC tumor bearing animals (Fig.2B,inset). Fig.3 confirms the zinc trafficking dysregulation where immunohistochemistry of these animals indicate the increase in import transporter ZIP3 concentrated mostly in the tumor parenchyma and surrounding pancreatic tissue.Discussion and Conclusion
Recently, we discovered that glucose stimulates the secretion of zinc from prostate cells, and that zinc is markedly decreased in prostate cancer while remaining elevated in benign conditions. This paradigm of stimulating zinc secretion by an exogenous agent had only been observed with glucose and useful to detect prostate cancer. 9-10 In the exocrine pancreas, zinc is packaged in granules along with zymogen enzymes, and functional receptors that mediate zymogen secretion have been identified for cholecystokinin, acetylcholine, gastrin releasing peptide, substance P, vasoactive intestinal peptide, and secretin. These agonists act on their respective G-protein-coupled receptors (GPCRs) located in the basolateral membrane and stimulate the secretion of zymogen granules docked on the luminal side. Caerulein, a surrogate for cholecystokinin, activates its respective GPCR and act via Ca2+ movement triggering depolarization-stimulated exocytosis and thus zinc secretion. By administering supraphysiological levels of caerulein in combination with extracellular zinc probes, it is possible to image exocrine function and to indirectly interrogate the dysregulation of zinc transporters in the malignant exocrine pancreas non-invasively by MRI. These changes in exocrine function may provide a glimpse at early malignant transformations disease as it was observed in the PDAC cells and stroma of animals bearing tumor cells as early as 1 week. These results highlight the importance of metal homeostatic dysregulation and their role in secretory tissue carcinogenesis.Acknowledgements
We acknowledge, Christian Preihs and A.Dean Sherry for kindly providing the Gd-based zinc probes.References
1. Oberstein, P. E.; Olive, K. P., Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol 2013, 6 (4), 321-37.
2. Zhang, Q.; Zeng, L.; Chen, Y.; Lian, G.; Qian, C.; Chen, S.; Li, J.; Huang, K., Pancreatic Cancer Epidemiology, Detection, and Management. Gastroenterol Res Pract 2016, 2016, 8962321.
3. Kelleher, S. L.; McCormick, N. H.; Velasquez, V.; Lopez, V., Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2011, 2 (2), 101-11.
4. Costello, L. C.; Franklin, R. B., A Review of the Current Status and Concept of the Emerging Implications of Zinc and Zinc Transporters in the Development of Pancreatic Cancer. Pancreat Disord Ther 2013, Suppl 4.
5. Costello, L. C.; Zou, J.; Desouki, M. M.; Franklin, R. B., Evidence for changes in RREB-1, ZIP3, and Zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer 2012, 43 (4), 570-8.
6. Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J. P.; Chang, S. M.; Cousins, R. J.; Fisher, W. E.; Brunicardi, F. C.; Logsdon, C. D.; Chen, C.; Yao, Q., Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A 2007, 104 (47), 18636-41.
7. Martins, A. F.; Clavijo Jordan, V.; Bochner, F.; Chirayil, S.; Paranawithana, N.; Zhang, S.; Lo, S. T.; Wen, X.; Zhao, P.; Neeman, M.; Sherry, A. D., Imaging Insulin Secretion from Mouse Pancreas by MRI Is Improved by Use of a Zinc-Responsive MRI Sensor with Lower Affinity for Zn(2+) Ions. J Am Chem Soc 2018, 140 (50), 17456-17464.
8. Erstad, D. J.; Sojoodi, M.; Taylor, M. S.; Ghoshal, S.; Razavi, A. A.; Graham-O'Regan, K. A.; Bardeesy, N.; Ferrone, C. R.; Lanuti, M.; Caravan, P.; Tanabe, K. K.; Fuchs, B. C., Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy. Dis Model Mech 2018, 11 (7).
9. Clavijo Jordan, M. V.; Lo, S. T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W. H.; De Leon-Rodriguez, L. M.; Lubag, A. J.; Rofsky, N. M.; Sherry, A. D., Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc Natl Acad Sci U S A 2016, 113 (37), E5464-71.
10. Clavijo Jordan, V.; Al-Ebraheem, A.; Geraki, K.; Dao, E.; Martins, A. F.; Chirayil, S.; Farquharson, M.; Sherry, A. D., Synchrotron Radiation X-ray Fluorescence Elemental Mapping in Healthy versus Malignant Prostate Tissues Provides New Insights into the Glucose-Stimulated Zinc Trafficking in the Prostate As Discovered by MRI. Inorg Chem 2019, 58 (20), 13654-13660.
Figures