3064
In vivo three-dimensional evaluation of hypoxia in nasopharyngeal carcinomas using FMT-CT and MSOT1the First Affiliated Hospital,Jinan University, Guangdong, China, 2Institute of Automation, Chinese Academy of Sciences, Beijing, China
Synopsis
The accurate imaging hypoxia is especially vital for nasopharyngeal carcinoma (NPC) patients undergoing radiotherapy. Multimodality molecular imaging have great potential to acquire hypoxic imaging with high sensitivity and more accuracy. We propose a novel imaging strategy,which combined the hybrid fluorescence molecular tomography-computed tomography (FMT-CT) and the multispectral optoacoustic tomography (MSOT), for achieving three-dimensional (3D) quantitative evaluation of NPC hypoxia in small animal models.The results could not only detect the hypoxia in small sizes of NPCs and lymph nodes metatasis, but also visualize the heterogeneity of hypoxia in 3D.
Synopsis
The accurate imaging hypoxia is especially vital for nasopharyngeal carcinoma (NPC) patients undergoing radiotherapy. Multimodality molecular imaging have great potential to acquire hypoxic imaging with high sensitivity and more accuracy. We propose a novel imaging strategy,which combined the hybrid fluorescence molecular tomography-computed tomography (FMT-CT) and the multispectral optoacoustic tomography (MSOT), for achieving three-dimensional (3D) quantitative evaluation of NPC hypoxia in small animal models.The results could not only detect the hypoxia in small sizes of NPCs and lymph nodes metatasis, but also visualize the heterogeneity of hypoxia in 3D.Purpose
The aim of this study was to propose a novel imaging strategy for achieving three-dimensional (3D) quantitative evaluation of NPC hypoxia in small animals.Methods
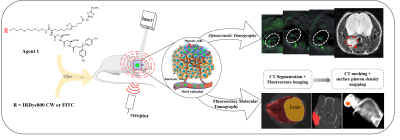
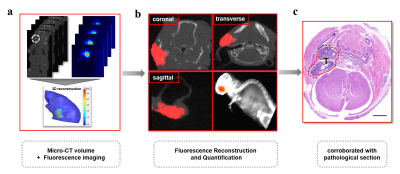
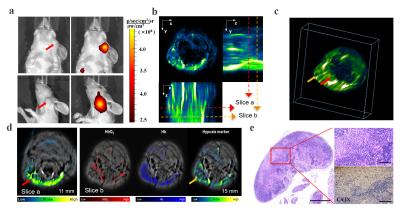
For designing the hypoxia targeting probe, we chose carbonic anhydrase IX (CAIX) as our targeting cellular marker, whose expression is exclusively upregulated by activated hypoxia-inducible factor-1a (HIF-1a). It has been proven to be one of the biomarkers for hypoxia in head and neck tumors, as well as a robustly negative prognostic marker for NPCs. Therefore, a CAIX-specific molecular probe named CAIX-800 was developed for hypoxic imaging. NPC subcutaneous, orthotopic(at the early stage of NPC, 2 weeks after injection), and spontaneous lymph nodal metastasis mouse models(at advanced stage of NPC, 6 weeks after injection) (n = 5 mice per group) were established for accessing the imaging strategy. A multi-modality imaging method, which combined the hybrid fluorescence molecular tomography-computed tomography (FMT-CT) and the multispectral optoacoustic tomography (MSOT) were conducted for 3D quantitative evaluation of tumor hypoxia. The strengths and weaknesses of each modality, as well as the benefits of combining both approaches in evaluating NPC hypoxia, were illustrated in different perspectives. MRI, histological analysis, and immunohistochemical analysis were employed as references for comparison and validation.Results
At the earlier stage of NPC, the highly sensitive FMT-CT enabled a precise 3D localization of the hypoxia biomarker with high contrast. At the advanced stage, MSOT provided a multispectral analysis of the biomarker and hemoglobin with high resolution. The combination of high sensitivity and high resolution from FMT-CT and MSOT could not only detect the hypoxia in small size of NPCs, but also visualize the heterogeneity of hypoxia in 3D.Discussion
This study demonstrated a FMT-CT and MSOT multi-modality imaging strategy designed for 3D quantitative evaluation of hypoxia in orthotopic NPC bearing mouse models. We chose CAIX as the molecular marker of NPC hypoxia and synthesized the low-molecular-weight ligand CAIX-800 as the probe for both fluorescence and optoacoustic tomographic imaging. The hypoxia in orthotopic NPC and lymph node metastatsis bearing mouse models can be quantitatively evaluated in 3D by using the multimodality imaging strategy.Conclusion
The integration of high sensitivity and high resolution from different imaging modalities achieved a comprehensive and quantifiable hypoxia visualization in different stages of NPC development. These findings may potentially benefit the radiotherapy of NPC patients in the future.Acknowledgements
We thank all participants for their endeavor and contribution in this study. We thank the Institute of Automation ChineseAcademy of Sciences and the Peking University First Hospital for their support of this research project.References
1.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410.
2. Hui EP, Chan ATC, Pezzella F, Turley H, To KF, Poon TCW et al. Coexpression of Hypoxia-inducible Factors 1α and 2α, Carbonic Anhydrase IX, and Vascular Endothelial Growth Factor in Nasopharyngeal Carcinoma and Relationship to Survival. Clinical Cancer Research 2002;8:2595-604.
3. Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727-35.
4. Hui EP, Chan ATC, Pezzella F, Turley H, To KF, Poon TCW et al. Coexpression of Hypoxia-inducible Factors 1α and 2α, Carbonic Anhydrase IX, and Vascular Endothelial Growth Factor in Nasopharyngeal Carcinoma and Relationship to Survival. Clinical Cancer Research 2002;8:2595-604.
5. Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674-87. 6. Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L et al. Expression of HIF-1alpha and CAIX in nasopharyngeal carcinoma and their correlation with patients' prognosis. Med Oncol. 2014;31:304.
Figures