3063
Magnetic Resonance Imaging of Single-Cell Dynamics in the Olfactory Bulb
Nikorn Pothayee1, Stephen Dodd1, Gary Zabow2, and Alan Koretsky1
1Laboratory of Functional and Molecular Imaging, National Institutes of Health, Bethesda, MD, United States, 2Magnetic Imaging Group, National Institute of Standards and Technology, Boulder, CO, United States
1Laboratory of Functional and Molecular Imaging, National Institutes of Health, Bethesda, MD, United States, 2Magnetic Imaging Group, National Institute of Standards and Technology, Boulder, CO, United States
Synopsis
There is great potential if single cells can be tracked in vivo in the mammalian brain. Here, we demonstrate the applicability of microfabricated gold-coated iron particles for in vivo tracking of single-cell dynamics. These particles have a pure iron core as opposed to the more commonly used iron-oxide based particles. Following in situ labeling of neural precursor cells, we show that the migration of individual cells can be visualized in real-time.
Introduction
In mammalian brains, new interneurons are continually added to the OB from the subventricular zone (SVZ) via the rostral migratory stream (RMS). These precursor cells differentiate into new neurons, which are thought to be involved with olfactory processing and learning. Over the past decade, MRI has emerged as a complementary tool to visualize the migration of the precursor cells in vivo [1]. The method involves in situ labeling with micron-sized iron oxide particles (MPIOs), which enables the labeled cells to be detected by T2*-weighted MRI. Using this technique, pattern and speed of the migration have been measured [2, 3]. However, MRI investigation into migratory behavior of an individual cell remains difficult due to the sensitivity limit of MPIOs. In this work, we seek to enhance detectability of single cell by employing microfabricated gold-coated iron particles as contrast agent. We demonstrate that these particles have greater sensitivity than MPIOs and can be used to label neural precursor cells in vivo and enable real-time tracking of individual cells in the brain of live animals.Methods
Gold-coated iron particles were microfabricated onto glass wafers in a manner similar to the method of [4]. The diameter of each particle was 1 um at the base with a thickness of ~200 nm. An electron micrograph showing example particles is shown in Figure 1A. The full wafer was sectioned into 5 x 25 mm strips, with a yield of ~ 12 million particles per strip . The particles may be released by removing the sacrificial copper layer with a copper etchant. To demonstrate in vivo MRI labeling feasibility, the particles are washed and suspended in saline for injection into the lateral ventricle near subventricular zone (SVZ) niche of the rat brain. MRIs for phantom and in vivo images were acquired in an 11.7T scanner. In vivo 3D-gredient echo MRI were performed at 1 and 2 weeks post-injection. Parameters were TR/TE = 30/10 ms, with a 60-mm isotropic resolution, scan time = 19 min.Results and Discussion
The gold-coated iron particles were fabricated with uniform sizes (Figure 1A-C). These particles have high moment and caused the signal drop, at 50-mm isotropic resolution, greater than 80%, which is superior to MPIOs (Bang’s Laboratory with 1.6 mm particles) which have average of 30% signal drop at same resolution. The particles were further modified with fluorescence probe AlexaFlour 647 for histological confirmation (Figure 1D-E). Following injection into the SVZ of adult rats, these particles could be internalized by the precursor cells. The migration along the RMS into the OB can be readily visualized by MRI. Immuno-staning with doublecortin (DCX), a marker for migrating immature neurons, confirmed the uptake of the particles (Figure 2). In the OB, average signal drop caused by microfabricated particles was approximately 75% which is consistent with the in vitro measurement (Figure 3). In contrast, the signal drop caused by MPIOs was approximately 30% due to their non-uniform sizes and varying degree of iron content [3]. Thus, the microfabricated iron particles indeed has superior sensitivity than MPIOs in detecting single cells. MRI at different time points revealed migration of the cells into the outer layer of the olfactory bulbs, which occurs between 1 and 2 weeks after birth of the precursor cells and their exit from the SVZ (Figure 4). Finally, continuous MR imaging for 4 hours (19 min temporal resolution) could capture real-time movement of the individual cells (Figure 5).Conclusion
We have shown that microfabricated iron particles can be used for in vivo cell tracking experiments in the rat brain. The particles are superior to the widely-used MPIOs in terms of sensitivity. Moreover, our results show that high moment magnetic particles can enable the detection of real-time migration of individual cells. Ability to measure the dynamics of migration at single-cell level could provide more accurate details of how external stimuli and activity affect behavior of neural precursor cells in the brain.Acknowledgements
This research was supported by the NINDS Intramural Research Program of NIH. We would like to thank the Mouse Imaging Facility at NIH and to NIST at Boulder, Colorado for access to their facility.References
[1] Shapiro E.M., Gonzalez-Perez O., Garcia-Verdugo J. M., et al., 2006, Neuroimage, 32, 1150-1157. [2] Nieman, B.J., Shyu, J.Y., Rodriguez, J.J., et al., 2010. Neuroimage, 50, 456–464. [3] Pothayee N., Cummings D.M., Schoenfeld T. et al., 2017, Neuroimage, 158, 232-241. [4] Zabow G., Dodd S. J., Shapiro E. M. et al., Magn. Reson. Med., 65, 645-655.Figures
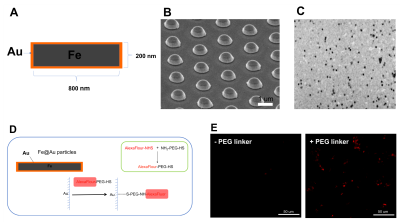
Figure 1. Fabrication
and functionalization of gold-coated iron particles. A) Structure of particle.
B)SEM image of particles as fabricated onto the wafer C) 50-mm
isotropic resolution MR image of released particles suspended in agarose phantom.
C) Surface modification of particles with PEG linker and fluorescent dye D)
Fluorescent images of particles following surface modification with AlexaFlour
647 (red).
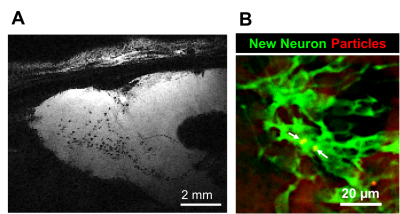
Figure 2. In situ
cell labeling of neural precursor cells in the adult rat brains. A) Parasagittal
view of the gradient-echo MR image of the rat brain at 1 week following
injection of microfabricate particles into the SVZ. Labeled-cells are seen as
hypointense dots along the RMS and in the OB. B) Fluorescent image of the neural
precursors (DCX, green) that contain particles (red) in the OB.
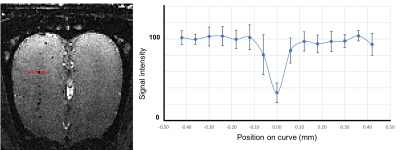
Figure 3. Average
signal intensity drop generated from the particles in the OB.
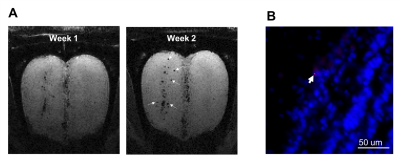
Figure 4. Serial
imaging at 1 and 2 weeks post-injection of particles into the SVZ show radial
migration of the labeled cells. A) Coronal views of the MR images of the OB
from the same animal at 1 and 2 weeks post-injection. The labeled cells (dark
spots, arrowed) could be seen at outer layer (superficial layer) of the OB at 2
weeks but not as 1 week. B) Histological
confirmation of the presence of the particles (red) in the mitral cell layer
(outer layer) of the OB.

Figure 5. Real-time
imaging of single-cell migration. Arrow indicates migrating cell moving along y-axis
from the starting position (dotted line). Images were acquired with 19 min temporal resolution during 4-hour imaging session.