3062
The utility of trastuzumab-based imaging agents for the development of theranostic for breast cancer1Department of Radiology, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing, China, 2National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, 3GE healthcare, China, Beijing, China
Synopsis
Superparamagnetic iron oxide nanoparticles (SPIONs) has been applied in diagnosis of different cancers, however its potential in breast cancer has not been fully explored. In this study, we research into the value of SPIONs conjugated with trastuzumab and indocyanine green (ICG) in becoming an effective multifunctional imaging agent and a tool for photothermal therapy for HER2-positive breast cancer. It was found that the biodistribution of dual-modality imaging agents has been observed by MRI and fluorescence imaging, which could provide effective technical assistance for HER2-positive breast cancer treatment.
Purpose
To investigate the capability of Fe3O4-trastuzumab-ICG nanoparticles (NPs) to target human epidermal growth factor receptor 2 (HER-2) receptors on breast cancer cells.Methods
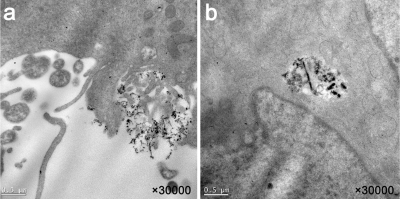
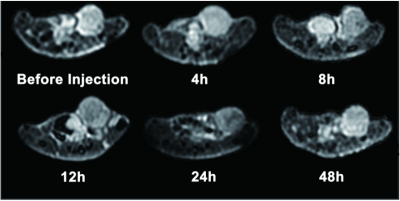
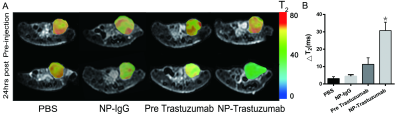
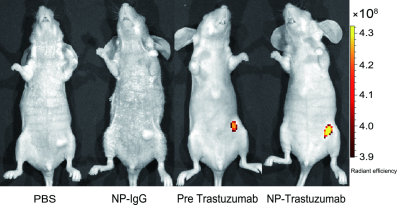
Superparamagnetic iron oxide nanoparticles (SPIONs) conjugated with trastuzumab and indocyanine green (ICG) were synthesized. A series of characterization of NPs were tested. Internalization was assessed on HER-2 positive breast cancer cells SK-BR-3 cells by transmission electron microscopy (TEM). The biodistribution of dual-modality imaging agents was observed by magnetic resonance imaging (MRI) and fluorescence imaging in a SK-BR-3 xenograft model to evaluate the targeting effects of NPs in vivo. Additionally, the photothermal effects of NPs was evaluated.Result
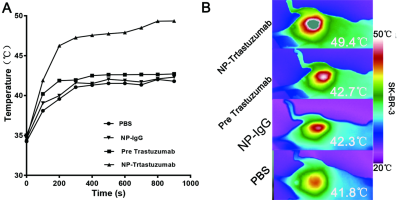
The Fe3O4-trastuzumab-ICG NPs exhibited ideal physicochemical properties. The TEM images revealed that the NPs could target SK-BR-3 cells and then enter the endoplasmic reticulum. MRI studies showed a marked uptake of NPs in tumor region with decreased T2 relaxation time, particularly at 24 h post-injection. Moreover, ΔT2 values (average pre-injection T2–average 24 h post-injection T2) of the tumor region in Fe3O4-trastuzumab-ICG group (30.7±4.8) ms were observed to be higher, and these differences were statistically significant (P<0.05) compared to PBS group (3.1±1.1) ms, Fe3O4-IgG-ICG group (4.4±0.9) ms and trastuzumab + Fe3O4-trastuzumab-ICG group (11.3±3.8) ms. Fluorescence imaging studies revealed that tumor-specific accumulation of ICG was observed in Fe3O4-trastuzumab-ICG group at 24 h following intravenous administration. Furthermore, the Fe3O4-trastuzumab-ICG NPs were shown to generate photothermal effect in tumor site, the maximum temperature reached 49.4℃ after continuous irradiation of near infrared light.Discussion
HER-2 positive breast cancer is usually associated with poor prognosis, with great possibility of recurrence. Accordingly, assessment of the expression of HER-2 is a significant step for accurate diagnosis and treatment[1]. Trastuzumab is a common targeted drug in the standard-of-care treatment for HER-2 positive breast cancers[2]. One possible approach to increasing the circulation time of NPs in the blood stream is to modify the NPs with polyethylene glycol (PEG) films[3]. The size and zeta potential of NPs have significant role in non-specific uptake and tumor targeting. NPs between the size of 10 – 100 nm in diameter are desirable since they are too large to be filtered out by the kidneys but small enough to not be recognized by the meticuloendothelial system for elimination[4,5]. It is really essential to prepare NPs with relative high r2 so that a little dose of NPs is well utilized to obtain satisfied imaging result. Hence, the NPs were potential candidates as MRI contrast agents[6]. Previous study confirmed that irreversible tumor damage would be induced when temperature reached over 43 ℃[7]. The maximum temperature of Fe3O4-trastuzumab-ICG group, 49.4 ℃, exceeding the damage threshold, while that of the other three groups would be insufficient to achieve tumor destruction. MRI is an effective noninvasive means for imaging cancer due to improved spatial resolution and deep penetration depth[8-10]. Owing to the unique properties of some fluorophore, which can absorb near-infrared laser and convert it to heat, destroying cancer cells[11-14]. We have studied the application possibility of ICG on breast cancer. It is noteworthy that dual-modality assembled NPs have complicated structures with large size, which results in rapid hepatic and renal clearance. Additionally, it may be relatively expensive, which limits its application in clinic.Acknowledgements
This work was financially supported by National Basic Research Program of China (973 Program, 2014CB744505). The authors wish to acknowledge and thank Xukun Li for providing technical assistance.References
[1]Mihaly Z, Gyorffy B. [HER2-positive breast cancer: available targeted agents and biomarkers for therapy response][J]. Magy Onkol,2013,57(3):147-156.
[2] Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect[J]. Adv Drug Deliv Rev,2011,63(3):131-135.
[3] Zhang M, Zhang Y, Kohler N. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake[J]. 2002,23(7):1553-1561.
[4] Veiseh O, Gunn J W, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging[J]. Adv Drug Deliv Rev,2010,62(3):284-304.
[5] Alexis F, Pridgen E, Molnar L K, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles[J]. Mol Pharm,2008,5(4):505-515.
[6] Corot C, Robert P, Idee J, et al. Recent advances in iron oxide nanocrystal technology for medical imaging☆[J]. Advanced Drug Delivery Reviews,2006,58(14):1471-1504.
[7] Zhao P, Zheng M, Yue C, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles[J]. Biomaterials,2014,35(23):6037-6046.
[8] Lauterbur P C. Image formation by induced local interactions. Examples employing nuclear magnetic resonance. 1973[J]. Clin Orthop Relat Res,1989(244):3-6.
[9] Fox M D, Raichle M E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging[J]. Nat Rev Neurosci,2007,8(9):700-711.
[10] Major J L, Meade T J. Bioresponsive, cell-penetrating, and multimeric MR contrast agents[J]. Acc Chem Res,2009,42(7):893-903.
[11] Liang C, Diao S, Wang C, et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes[J]. Adv Mater,2014,26(32):5646-5652.
[12] Cheng L, Yang K, Li Y, et al. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy[J]. Biomaterials,2012,33(7):2215-2222.
[13] Guo C, Yu H, Feng B, et al. Highly efficient ablation of metastatic breast cancer using ammonium-tungsten-bronze nanocube as a novel 1064 nm-laser-driven photothermal agent[J]. Biomaterials,2015,52:407-416.
[14] Huo D, He J, Li H, et al. X-ray CT guided fault-free photothermal ablation of metastatic lymph nodes with ultrafine HER-2 targeting W18O49 nanoparticles[J]. Biomaterials,2014,35(33):9155-9166.
Figures