3057
TSPO-PET/MRI Reveals Increased Neuroinflammation in Basal Ganglia of Chronic Fatigue Syndrome Patients1Bioengineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Medicine, Stanford University, Stanford, CA, United States, 4Neurology, Stanford University, Stanford, CA, United States
Synopsis
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating disease affecting millions of people in the United States alone, but little is known about the underlying pathophysiology. We show that simultaneous TSPO-PET/MRI measurements using [11C]DPA-713, including SUV, SUVr, QSM, R2*, and volume can uncover new information about this disease. We find that the putamen has significantly higher TSPO-PET signal in ME/CFS subjects compared to healthy controls, indicating an elevated inflammatory response in this area. This finding corresponds to previous fMRI and diffusion imaging findings and may help with future diagnosis and tracking of this chronic, widespread disease.
Introduction
ME/CFS is a complex and perplexing disease, considered one of the biggest medical and scientific challenge of the 21st century. In the United States alone, approximately 1–2.5 million people suffer from this disease. Clinical presentation includes chronic fatigue not alleviated by rest (> 6 months), as well as pain and cognitive symptoms. No FDA-approved disease-modifying treatment is available. Theories posit that ME/CFS may be triggered by a virus which results in systemic inflammation affecting several parts of the body over many years, but few studies have attempted to measure inflammation at the molecular level in the central nervous system of these patients. There is an urgent unmet need to identify and validate techniques that enable early and accurate detection of the molecular underpinnings of ME/CFS so that we can better diagnose and evaluate potential treatments. We assess the utility of positron emission tomography targeting the translocator protein 18kDa (TSPO-PET) for detecting and tracking the inflammatory component of ME/CFS in the brain, and we investigate whether this signal corresponds with disease severity and with quantitative susceptibility mapping (QSM) using simultaneously-acquired magnetic resonance images (MRI).Methods
A total of 9 subjects, 4 CFS and 5 age/sex-matched healthy controls, underwent a dynamic 60-minute TSPO-PET scan on a 3T PET-MR (SIGNA, GE, WI, USA) using time-of-flight capability following a 15mCi intravenous injection of [11C]DPA-713 (chemical and radiochemical purity >99%, molar activity 2–9 Ci/mmol). During the PET acquisition a sagittal T1-weighted inversion recovery spoiled gradient echo (TR 7.6ms, TE 3.1ms, FA 11, 1x1x1.2mm resolution, 5m46s scan time), and an axialQSM (12 echoes, TE 3.1ms, TR 65.1ms, FA 15, 0.93x0.93x1.6mm resolution, 8m13s scan time) series were acquired. Standardized uptake value (SUV) maps were calculated from 30–60 minute summed PET data and normalized to a pseudo-reference region for each subject (cerebellar cortex). SUV, SUVr maps, QSM and R2* images were precisely registered to coronal T2 MR image space using the T1-w IRFSPGR as an intermediate registration volume using niftireg. FreeSurfer 6.0 was used to segment the entire brain, and regions of interest were grouped into larger subregions for statistical evaluation. Parotid glands were manually segmented for each subject, and SUV was quantified. QSM and R2* maps were calculated using MEDI. SUVr, QSM, R2*, and volume were quantified. ME/CFS subjects were compared to healthy controls using a linear regression model with age and TSPO polymorphism/rs6971 genotype as regressors in PET-based metrics, age and head size as regressors in volume measurements, and only age as a regressor in QSM-based metrics. Only high and mixed-affinity binders were included in SUVr analysis.Results
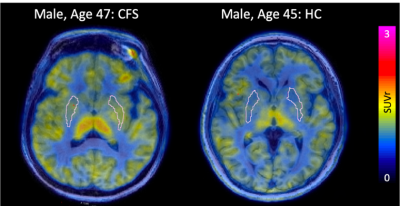
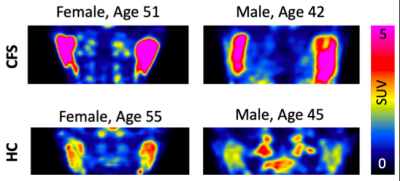
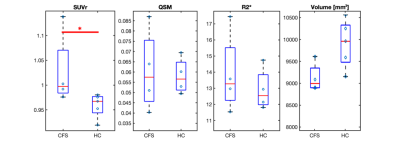
ME/CFS patients had a statistically higher SUVr in the putamen (p=0.035) compared to healthy controls (Figure 1). We also found a trend toward significantly higher SUV in the parotid glands of ME/CFS patients (p=0.069) (Figure 2) and a trend toward significantly smaller volume in the putamen of ME/CFS patients compared to healthy controls (p=0.088). There was no statistically significant QSM or R2* difference in the putamen between the two groups (Figure 3).Discussion
The putamen, part of the basal ganglia, is associated with regulating complex movements, and inflammation in this region could be related to patient fatigue. Other studies have found low fMRI activity in the putamen of children with ME/CFS1 and larger mean diffusivity in the basal ganglia of adolescents with ME/CFS2. The trend in the parotid glands suggests we may identify systemic markers of inflammation using PET.Conclusion
Increased [11C]DPA-713 uptake in the putamen of ME/CFS patients compared to healthy controls suggests that chronic neuroinflammation in this brain region may be involved in the initiation and progression of this disease. Taken together, TSPO-PET/MRI appears to be a promising technique for further investigating ME/CFS pathophysiology and improving diagnosis and management of these patients.Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship.References
1. Mizuno, K., Kawatani, J., Tajima, K., Sasaki, A. T., Yoneda, T., Komi, M., … Watanabe, Y. (2016). Low putamen activity associated with poor reward sensitivity in childhood chronic fatigue syndrome.NeuroImage. Clinical, 12, 600–606. doi:10.1016/j.nicl.2016.09.016
2. Nakagawa S, Takeuchi H, Taki Y, Nouchi R, Kotozaki Y, Shinada T, et al. (2016) Basal ganglia correlates of fatigue in young adults. Nature Scientific Reports, 6:21386. https://doi.org/10.1038/srep21386
Figures