3054
Three compartment model approach to acquire kinetic information of reactive contrast agent1Bio and Brain Engineering, KAIST, Daejeon, Korea, Republic of, 2Biological Science, KAIST, Daejeon, Korea, Republic of
Synopsis
Common contrast enhanced MRI study relies on low retention and inert contrast. With the emerging trend of using paramagnetic metal for theragnostic study, few nanoparticles have been reported to have T1 enhancement property. In this research, we try to model the kinetics of such nanoparticles in mouse with A549 tumor. A novel three compartment model were introduced to explain the behavior of Mn-BRNP nanoparticles within mouse blood circulation system. This is the first study to explore reactive contrast agent with high retention, and successfully derived kinetic parameters that characterize the drug uptake behavior.
Introduction
New class of cancer-treating drug study has been concentrated on using paramagnetic metal such as Mn and Fe as substrate to the drug molecule. These metals possess T1/T2 enhancement properties that can be observed in MRI scan. Despite its main purpose as therapeutic agent, the magnetic properties can potentially be used to characterize the hemodynamics of the subject. This research aims to provide a model that explains drug transport within organs and tissues by observing the T1-weighted MRI image dynamically. As such, treatment using these agents may potentially provide information on tumor anatomy and nutrient uptake rate.Contrast Agent Material
A class of manganese-chelated bilirubin nanoparticle (Mn-BRNP) were used as contrast agent and tumor therapeutic agent in this study. The nanoparticles were in bilirubin shape when dormant and deformed when in contact with reactive oxygen species (ROS), dispersing manganese particles which further reduced T1 time. This effect was confirmed by T1 mapping using IRSE sequence.MRI Signal Enhancement
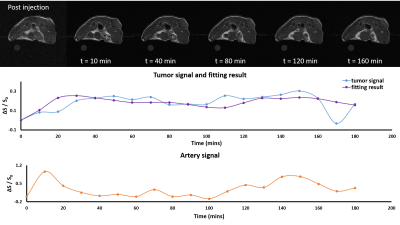
Signal enhancement were observed in 3T MRI (MRS 3000, MR solutions) using T1-weighted spin echo sequence for 5 mice. Preliminary study showed the contrast agent retention to be longer than 24 hours. This allowed us flexibility in temporal resolution of the T1-weighted image scan. In this case, we used 256x256 matrix size, with TE/TR of 11/550ms. The scan was performed dynamically for 3 hours with 10‑minute temporal resolution, resulting in 18 time points post-injection. Nanoparticle Mn-BRNP was introduced into the mouse blood stream via ocular venous injection.The signal enhancement is assumed to be caused solely by the contrast agent arrival. This gives proportionality relationship of concentration and observed signal as follow: $$ c(t) = \frac{(s(t) - s_0)}{s_0}$$ where s0 is the pre-injection signal. Concentration was calculated from artery space and tumor site. The difference between concentration profile of artery and tumor site should be described by the proposed model.
Three Compartment Model
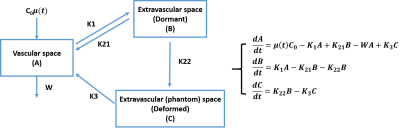
Conventional contrast agents commonly used in DCE or DSC MRI are nonreactive and have low retention in the body. Therefore, common DCE or DSC scan does not take more than one hour of scan time and only consider the first pass of tracer bolus for kinetics modeling. Further, the model used are mainly based on exchange between two compartments which are vascular and extravascular space. However, the contrast agent in this study would not have the same kinetics due to its reactivity and high retention. Instead, we added another compartment that served to differentiate the dormant and deformed contrast agents. Thus, we propose a three compartment model where the third compartment acts as an arbitrary space where nanoparticle deformation is analogous to compartment movement (Figure 1).Arterial input function (AIF) was acquired from abdominal artery while the tumor signal was acquired along multiple slices and averaged for each time point. The model took AIF as input and the output was compared to the observed tumor signal. The model had 5 parameters (k1, k21, k22, k3, w) to be estimated in order to produce desired output. Multi-parametric fitting were done with Monte Carlo approach in a wide range for each parameter. Fitting error were evaluated through the following range (k1 = .01 – 1, k21 = .01 – 1, k22 = .01 – 1, k3 = .0001 - .01, and w = .01 – 10). The difference between model output and observed tumor signal were calculated using squared error. The parameter set was determined by minimizing the squared error.
Results
The measured relaxivity of the nanoparticle were 3.1 mM-1/s when the nanoparticle was dormant, and 6.18 mM-1/s when deformed. The signal intensity changed in tumor area right after injection with peak intensity observed 150 mins after injection (Figure 2). Signal enhancement was observed in two stages indicating initial tracer arrival and further enhancement due to contrast agent deformation. Fitted results showed close correlation (correlation coefficient = 0.6). The fitting results across the 5 mice in the unit of min-1 were: k21 = 0.01 ± 0.002, k22 = 0.1 ± 0.04, k1 = 0.13 ± 0.007, k3 = 0.015 ± 0.005, and w = 5 ± 0.6.Discussion
The contrast agent is expected to circulate inside the mouse body for up to 24 hours before clearance. This means buildup of our nanoparticle will be a compounded effect of the nanoparticle bolus after multiple pass into tumor area. Further, since the nanoparticle is designed to be a therapeutic agent, washout via capillary and vein will not be as dramatic as the conventional contrast agent. Thus, we expect the modeling of this nanoparticle is not directly correlated to conventional perfusion parameters such as blood flow or blood volume. Rather, we expect to have our parameters are correlated to tumor nutrient uptake or drug delivery efficiency. Modeling the dynamic signal characteristics for injection of this kind of theragnostic contrast agent is new and it has been unexplored yet. This study would be one of the first trials to explore the signal dynamics of the theragnostic contrast agent in vivo using MRI. Furthermore, the reactive theragnostic contrast agent was modelled for the first time using three compartment model. Further study is necessary to prove and/or revise the model in various organs and conditions.Acknowledgements
No acknowledgement found.References
[1] Koh TS, Bisdas S, Koh DM, Thng CH. Fundamentals of tracer kinetics for dynamic contrast-enhanced MRI. J Magn Reson Imaging 2011;34(6):1262-76.
[2] Barnes SL, Whisenant JG, Loveless ME, Yankeelov TE. Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics 2012;4(3):442-78.
[3] Ingrisch M, Sourbron S. Tracer-kinetic modeling of dynamic contrast-enhanced MRI and CT: a primer. J Pharmacokinet Pharmacodyn 2013;40(3):281-300.
Figures