3052
Natural Abundance Deuterium (2H) MRI of the Brain
Joshua D Kaggie1, Mary McLean1, Rolf F Schulte2, Dimitri A Kessler1, Frances Henson3, Fiona J Gilbert1, Martin J Graves1, and Ferdia A Gallagher1
1Radiology, University of Cambridge, Cambridge, United Kingdom, 2GE Healthcare, Munich, Germany, 3Veterinary Medicine and Divisino of Trauma and Orthopaedic Surgery, University of Cambridge, Cambridge, United Kingdom
1Radiology, University of Cambridge, Cambridge, United Kingdom, 2GE Healthcare, Munich, Germany, 3Veterinary Medicine and Divisino of Trauma and Orthopaedic Surgery, University of Cambridge, Cambridge, United Kingdom
Synopsis
We demonstrate natural abundance deuterium imaging of the normal human brain in vivo, without the requirement of an injected deuterated molecule, and within a 10-minute chemical shift imaging acquisition.
Introduction
Deuterium (2H) has a 0.01% natural abundance, which is low in comparison to that of proton (1H), but comparable to the concentrations of other ions, such as intracellular 23Na. The resulting lower sensitivity of 2H-MRI compared to 1H-MRI has resulted in a paucity of research in this area. There has been a resurgence of interest in 2H-MRI with the advent of deuterium metabolic imaging (DMI) and the imaging of 2H-labelled glucose. 2H-labelled molecules that measure metabolic activity are particularly interesting for measuring the Warburg effect in brain tumours. Recent work has shown MRI of 2H-labelled water and [6,6′-2H2]glucose at concentrations as high as 5%. 2H-labelled glucose has been shown to metabolise into lactate and glutamine/glutamate in the normal human brain and in brain tumours. 2H-MRI with [6,6′-2H2]glucose has the potential to rival FDG-PET imaging without the need for ionising radiation (1).One of the challenges of DMI is that it is difficult to optimise and standardise 2H-MRI in the absence of endogenous signal. Here we demonstrate natural abundance 2H-MRI, which can be used for optimization and quality assurance of hardware, pulse sequence, and reconstruction methods. This work will also aid the acquisition of signal following the administration of 2H-labelled molecules.
Methods
We modified a 16-rung birdcage coil to resonate at 19.8 MHz for 2H-MRI (Figure 1) for a 3T MRI system (MR750, GE Healthcare, Waukesha, WI). The birdcage connected to a quadrature 2H transmit/receive switch (Clinical MR Solutions, Brookfield, WI). The 28 cm diameter birdcage included copper conductor rungs with 8 cm length, 1 cm width, and with 2 cm end-ring widths.We imaged natural abundance 2H in the brains of two cadaveric sheep and in three normal volunteers with 3D chemical shift imaging (CSI). Volunteer imaging occurred with informed consent and local ethical approval. The 3D density-weighted CSI sequence acquired either 2 averages of a 12x12x12 matrix size, 2405 FID acquisitions, scan time 9.5 minutes; or 1 average of a 16x16x16 matrix size, 5408 transients, scan time 10.8 minutes. Both used field-of-view = 240 mm3, TR = 120 ms, flip angle = 45°, bandwidth = ±2.5 kHz, with 512 spectral points between -128 and +128 ppm per voxel, and both were zero-filled once in the time domain and once spatially in the X and Y planes for analysis. For viewing images based on the maximal spectral intensity, an additional 4x4 interpolation was applied with 3-point spline interpolation using SciPy (2).
3D gradient echo T1-weighted images were also obtained with the 1H body coil without repositioning of the subjects.
Results
Figure 2 shows sample spectra from a 5x5x2 cm3 region in the center of the brain. The spectra are dominated by a central heavy water peak, and several spectra show a possible fat peak at around -4ppm relative to water.Figure 3 shows the maximum signal in two axial slices of a normal volunteer. The cerebral ventricles demonstrate hyperintensity.
Figure 4 shows a sagittal view of the brain with the voxel-wise mean of the CSI spectra, demonstrating good delineation of the brain in 3D.
Figure 5 shows the MRF-derived 1H density and relaxation maps, which should relate to the endogenous images obtained with 2H.
Discussion
Despite the low natural abundance of 2H, we demonstrated that endogenous 2H signal of normal brains can be imaged in vivo using MRI.This work demonstrates that the evaluation of 2H methods can be performed without the need for increased signal following the administration of 2H-labelled molecules. Natural abundance imaging simplifies the development and optimization of new methods, such as the evaluation of more sensitive radiofrequency coils.
Baseline measurements with natural abundance imaging also establish the feasibility of performing dynamic metabolic measurements. Administration of 2H-labelled molecules would significantly increase the signal-to-noise above this baseline, e.g., using [6,6′-2H2]glucose. Resolution of isotopically-labelled metabolite peaks from the nearby naturally abundant heavy water signal will require more careful optimization of field homogeneity than that achieved in the current study using only linear shim adjustment.
Conclusion
We have demonstrated natural abundance deuterium imaging in vivo in normal human brains, which can be useful for methodological evaluation of pulse sequences and radiofrequency coils, and for baseline measurements before dynamic imaging.Acknowledgements
This work acknowledges support from the National Institute of Health Research Cambridge Biomedical Research Centre, Addenbrooke's Charitable Trust, Cancer Research UK, and from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 761214.References
1. De Feyter HM, Behar KL, Corbin ZA, Fulbright RK, Brown PB, McIntyre S, Nixon TW, Rothman DL, de Graaf RA. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Science advances 2018;4(8):eaat7314.
2. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J. SciPy 1.0--Fundamental Algorithms for Scientific Computing in Python. arXiv preprint arXiv:190710121 2019.
Figures

Figure 1 The 2H
deuterium coil in a 3T MRI system with a water (1H2O) phantom inside.
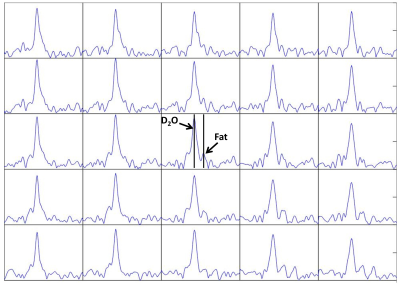
Figure 2. Spectral
data from 5x5 voxels of a normal human brain, interpolated voxel size
1x1x2cm. The spectra show a strong
central 2H2O peak as well as a peak which is likely to
represent fat at approximately -4 ppm from water.
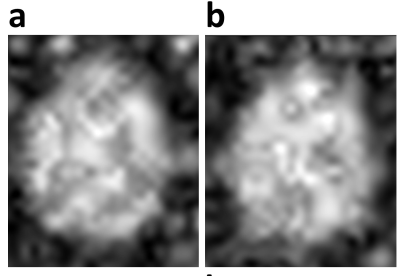
Figure 3. Two slices of natural
abundance 2H2O in a normal human brain, showing maximal
values of the spectra.

Figure 4. A single sagittal
slice of the same volunteer brain, with the mean spectral 2H
signal.
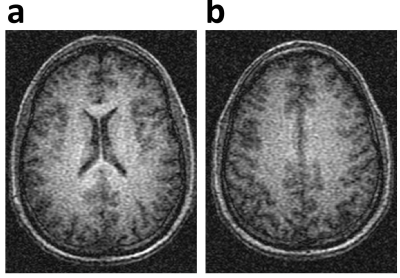
Figure 5. 3D gradient echo images of the same volunteer
in Figs 2-4, as obtained with the 1H body coil.