3049
New class of biodegradable responsive phosphorus–containing contrast agent for 1H/31P MR1Department of Computed Tomography, Magnetic Resonance and Clinical Experimental Spectroscopy, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2First Faculty of Medicine, Institute of Biophysics and Informatics, Charles University, Prague, Czech Republic, 3Supramolecular polymer systems department, Institute of Macromolecular Chemistry, Prague, Czech Republic, 4Faculty of Mechatronics Informatics and Interdisciplinary Studies, Technical University of Liberec, Liberec, Czech Republic, 5Faculty of Sciences, Department of Physical and Macromolecular Chemistry, Charles University, Prague, Czech Republic
Synopsis
We present a new class of biodegradable responsive 31P phosphorus–containing contrast agent for dual 1H/31P MRI. Both 1H and 31P MR imaging modalities offer complementary information and can be easily combined at the same experiment. The switch to phosphorus MR signal can reflect biochemical changes in the organism. An implementation for 1H/31P MR at 4.7 T is presented and its properties are investigated.
Introduction
Development of bimodal 1H/31P magnetic resonance responsive probe reflecting anatomy and biochemical activity of environment at the same time is extremely challenging2. The advantage of these modalities is that they can provide complementary information about physiological conditions in living tissue.The aim of this project is to develop and test a conceptually new class of biodegradable responsive phosphorus–containing contrast agent for 1H/31P MR. Presented probe is based on phytate (myo-inositol-1,2,3,4,5,6-hexakisphosphate) crosslinked with Ca2+ ions (CaIP6) and doped with different concentrations of Fe3+ ions. High content of phosphorus in phytate enables its detection by 31P MRS/MRI. Linked paramagnetic Fe3+ ions broaden the phosphorus signal, making it invisible on 31P MR and act as a probe for 1H MR only. When the link is broken and Fe3+ is cleaved out, the 31P signal appears due to its narrowing in the spectrum. This detachment can act as a response to external physiological changes (lower pH in cancer tissue1, bacteria producing iron chelating siderophores3 etc.). Here we present a pilot in vitro study focusing on the effect of linked iron on 31P MR signal and its chelation using iron-binding bacterial siderophore – deferoxamine (DFOA).
Methods
Presented nanoparticles (100–300 nm) are based on CaIP6 (phosphorus c= 1mM) doped with different concentrations of paramagnetic Fe3+ ions (iron c= 0, 0.68, 2.04, 2.73, 5.43, 13.6 mM,) linked by Ca2+ ions replacement. In vitro simulation of Fe3+ release was performed with DFOA (2.73 mM Fe3++DFOA; [DFOA]:[CaIP6] ratio 1:1)4, a compound possessing high affinity to Fe3+ ions. We chose 2.73 mM Fe3+ probe for testing, because of its 31P MR signal widening and adequate iron concentration to avoid over–chelation. Sufficiently high molecular weight of contrast agents (above renal threshold) assures sufficiently long blood circulation.Firstly probes were measured by 7 T 31P NMR for signal confirmation and then tested at 4.7 T scanner using self-made 1H/31P RF coil, which is wide tuned for both 1H and 31P nuclei, so that anatomical reference acquired by 1H MR could be obtained. 1H MR (FLASH, repetition time/echo time TR/TE=100/6 ms) was firstly applied for probes positioning. Probes MR properties were than assessed by 31P MRI/MRS. Single pulse sequence was used for obtaining 31P T1 relaxation times (TR=160–3000 ms, 3000 acquisitions) and for spectra comparison between probes containing different Fe3+ concentrations (TR=500 ms, scan time ST=16 h 40 m). For 31P MRI chemical shift imaging (CSI) sequence was optimized (TR=500 ms, ST=1 h, resolution 2.5x2.5x5.8 mm3). For visualization of Fe3+ influence on MR signal 1H MRI of phantoms was measured (T2-weighted imaging, TR/TE=2000/24 ms). Signal-to-noise ratio (SNR) was calculated from both 1H and 31P MR images and from 31P MR spectra. Pilot testing of highly concentrated 2.73 mM Fe3+ contrast cytotoxicity of probe was assessed using Alamar Blue assay (4T1 mammary carcinoma cell line; 48 h incubation).
Results
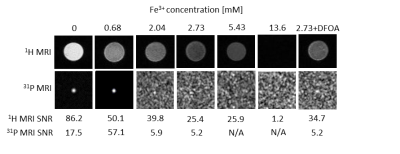
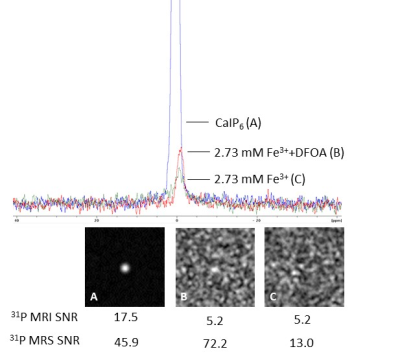
1H MRI of phantoms containing high amount of iron appear darker on T2-weighted images, with the lowest SNR=1.2 calculated from the probe doped with the highest Fe3+ concentration and the highest SNR=86.2 calculated for probe containing no iron. Effect of iron chelation was observed in 1H MRI (2.73 mM Fe3+ probe SNR=25.4; 2.73 mM Fe3++DFOA probe SNR=34.7) (Fig. 1). We confirmed linked iron ions impact on 31P MRS signal widening with increasing Fe3+ concentrations and no signal obtained from concentrations higher than 2.73 mM Fe3+. Influence of DFOA was visible in 31P MRS data of 2.73 mM Fe3++DFOA, showing signal increase (SNR=72.2) after ferric ions recomplexation by siderophore, comparing to 2.73 mM Fe3+ without DFOA (SNR=13) and to probe with no iron doping (SNR=45.9) (Fig.2). Signal restorations was also observed on 31P NMR, where a significant increase in signal intensity was observed in probe with DFOA. In 31P MRI signal was observed for low Fe3+ concentrations, with the highest SNR=57.1 for 0.68 mM Fe3+ (Fig. 1). 31P T1 relaxation times are decreasing with iron doping increase (0.68 mM Fe3+: T1=487 ms; 2.04 mM Fe3+: T1=305 ms). Cytotoxicity testing confirms that our probe is not significantly influencing cells viability.Discussion
Our results confirms the possible use of this new contrast agent as a 1H/31P MR responsive probe offering two imaging modalities. The processed data confirmed the effect of iron ions and iron chelating compounds (DFOA) on 31P MR signal, which changes can be used as a response for sensing early tissue pathology and biochemical processes in living organism. Small inconsistences in the SNR results can be explained due to the low sensitivity at magnetic field strength 4.7 T, however should not affect the detectability of 31P MR signal even in the lower magnetic fields used in clinical practice.Conclusion
We present bimodal 1H/31P MR responsive probe as the proof of principle. This new class of biodegradable responsive phosphorus-containing probe confirms sufficient sensitivity for exogenously triggered 31P MRI/MRS at 4.7 T. Our results indicates no cytotoxicity of testes probes; however further detailed testing on different cell lines should be performed.Acknowledgements
The study was supported by the Charles University, GA UK No 358119; Institute for Clinical and Experimental Medicine IKEM, IN00023001; Charles University, First Faculty of Medicine; Ministry of Education of the Czech Republic through the SGS project no. 21332/115 of the Technical University of Liberec.References
1. Kato Y, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013:13(89).
2. Yoo B and Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. 2008:13,1733-1752.
3. Miethke M and Marahiel M A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007:71(3):413–451.
4. Porter J, Viprakasit V. IRON OVERLOAD AND CHELATION. In:Cappellini MD, Cohen A, Porter J, et al. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. 3rd edition. Nicosia(CY):Thalassaemia International; 2014.
Figures