3047
Effective MR Molecular Imaging of Prostate Cancer with an EDB-Fibronectin-Specific Contrast Agent at Reduced Doses
Nadia Ayat1, Sarah Roelle1, Songqi Gao1, Yajuan Li1, and Zheng-Rong Lu1
1Case Western Reserve University, Cleveland, OH, United States
1Case Western Reserve University, Cleveland, OH, United States
Synopsis
This research highlights the potential of a targeted contrast agent specific to the extra-domain B (EDB-FN) to be utilized in the diagnosis of prostate cancer at reduced dosages. EDB-FN specific MR contrast agent MT218 produced robust tumor enhancement at dosages as low as 0.02 mmol Gd/kg.
Introduction
Gadolinium (III) based contrast agents (GBCAs) are commonly used for contrast enhanced MRI of cancerous lesions1. However, clinically used GBCAs are tumor-specific and unable to accurately detect and characterize aggressive tumors. In addition, repeated use of these agents at a relatively high dose may lead to adverse side effects including nephrogenic systemic fibrosis in patients with compromised renal function, and nonspecific brain accumulation. We have previously developed and characterized a targeted MRI contrast agent, named ZD2-N3-Gd(HP-DO3A) (MT218), that is specific to tumor extracellular matrix protein extradomain-B fibronectin (EDB-FN)2. MT218 produces superior enhancement in aggressive prostate cancer using 0.1 mmol Gd/kg, the clinical dosage for most GBCAs, and has shown the ability to differentiate aggressive prostate tumors from non-invasive tumors in mouse tumor models. We hypothesized that MT218 could be effective for magnetic resonance molecular imaging (MRMI) of aggressive tumors at substantially reduced doses due to its tumor specificity. This would significantly reduce the dose-dependent side effects related to GBCAs. In this study, we have evaluated the dosing effects of MT218 for tumor contrast enhancement in a rat tumor xenograft model with the dose as low as 0.02 mmol Gd/kg, only 1/5 of the clinical dose. To further evaluate the safety profile of MT218, we analyzed the biodistribution of MT218 for up to two weeks after intravenous administration.Methods
The contrast agent MT218 was provided by Molecular Theranostics LLC (Cleveland, OH). MRMI was conducted in male rats bearing PC3 human prostate tumor xenografts using a 3T MRS 3000 scanner (MR Solutions) with a rat short quad coil. Axial T1-weighted images were acquired pre and post-injection of MT218 at doses of 0.04 and 0.02 mmol Gd/kg using a fast spin echo (FSE) sequence with respiratory gating (TR = 305 ms, TE = 11 ms, FOV = 80 x 80 mm, slice thickness = 1 mm, Nav = 2, matrix = 128 x 128). The imaging protocol was repeated with the clinical agent ProHance at 0.1 mmol Gd/kg. Biodistribution studies were conducted to analyze the metabolic clearance and two-week tissue retention of MT218. Sprague dawley rats (5m + 5f, 7-8 weeks old) were randomly divided and injected with either MT218 or ProHance at a dose of 0.1 mmol Gd/kg via tail vein. The rats were placed in metabolic cages for 3 days, were feces and urine samples were collected at 8, 24, 48 and 72 hours post-injection. The rats were sacrificed at 14 days post-injection. Tissue samples, including brain, femur, heart, lung, liver, muscle, spleen and kidneys, were collected and weighed. The tissue samples were subsequently processed as previously reported, and Gd3+ concentration was measured by ICP-OES3.Results
Contrast-enhanced MRMI of PC3 tumor xenografts using MT218 produced strong signal enhancement at both subclinical doses of MT218. Figure 1A shows representative axial images at 10, 20 and 30 min post injection. Strong contrast enhancement was observed in the tumors with MT218 at both doses as early as 10 min post injection, persisting for at least 30 min. Much lower contrast enhancement was observed with the clinical agent ProHance. Quantitative analysis reveals that MT218 generates approximately 13-fold enhancement in rat tumors at 0.04 mmol Gd/kg (Figure 1B). MT218 at 0.02 mmol Gd/kg showed a dose-dependent decrease in contrast enhancement, but still exhibited up to 8-fold CNR increase. In contrast, clinical Gd(HP-DO3A) at 0.1 mmol/kg produced only a 3-fold CNR enhancement in the tumors. MT218 has no significant Gd(III) detected in tissues in rats 2 weeks post administration at a dose of 0.1 mmol/kg, same as the clinical agent ProHance.Discussion
Specific binding of MT218 to abundant EDB-FN in the extracellular matrix of aggressive PC3 human prostate tumor xenografts resulted in robust tumor enhancement in the aggressive prostate carcinomas for effective MRMI at reduced doses. MT218 at dosages as low as 0.02 mmol Gd/kg was able to produce more tumor enhancement than the clinical agent ProHance at a full dose. The results have validated our hypothesis and demonstrated the effectiveness of the MT218 for MRMI of aggressive tumors at subclinical doses. While administration of MT218 at the clinical dose reveals no significant long-term Gd accumulation, robust contrast enhancement of MT218 at a significant reduced dose will greatly minimize the potential toxic side effects of GBCAs. In conclusion, MT218 has the great promise for effective MRMI of aggressive prostate tumors at a substantially reduced doses with minimal dose-dependent side effects.Acknowledgements
This research was supported by the NIH grants R01CA211762 and R44CA199826.References
- Zhou, Z. & Lu, Z. R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5, 1-18, doi:10.1002/wnan.1198 (2013).
- Ayat, N. R. et al. Optimization of ZD2 Peptide Targeted Gd(HP-DO3A) for Detection and Risk-Stratification of Prostate Cancer with MRI. ACS Med. Chem. Lett. 9, 730-735, doi:10.1021/acsmedchemlett.8b00172 (2018).
- Han, Z. et al. Targeted Contrast Agent Specific to an Oncoprotein in Tumor Microenvironment with the Potential for Detection and Risk Stratification of Prostate Cancer with MRI. Bioconjug. Chem. 28, 1031-1040, doi:10.1021/acs.bioconjchem.6b00719 (2017).
Figures
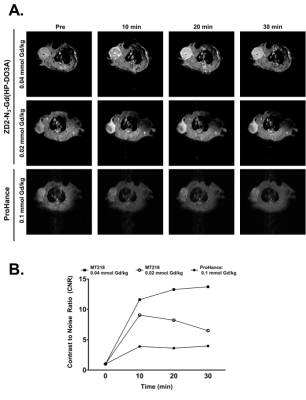
MRMI using MT218 shows strong tumor enhancement (A) at doses
of 0.04 and 0.02 mmol Gd/kg in PC3 prostate xenografts compared to clinical
control ProHance. Injection of 0.04 and 0.02 mmol Gd/kg MT18 results in a 13-and
8-fold CNR increase compared to ProHance, respectively (B).

Injection of MT218 yields no significant Gd(III)
detected in tissues in rats 2 weeks post administration at a dose of 0.1
mmol/kg, same as the clinical agent ProHance.