3046
A novel ANG-BSA/Car/ICG MNPs integrated for targeting therapy of glioblastoma1The First Affiliated Hospital of Shenzhen University, Shenzhen, China
Synopsis
Moat chemotherapeutic drugs failed to effectively treat glioblastoma (GBM) on account of the existence of blood-brain barrier, which play a role in GBM. In this study, we constructed an integrated nanoprobe based on albumin nanoparticles for targeted diagnosis and treatment of GBM. The nanoprobe consists of albumin-coated superparamagnetic iron oxide, Carmustine and indocyanine green to achieve bimodal imaging and drug delivery. And the surface-coupled Angiopep-2 can specifically bind to LRP, which is overexpressed in BBB and GBM cells. This novel targeting imaging and drug delivery system provides an efficient strategy for targeted therapy and intraoperative localization of GBM.
Introduction
Blood-brain barrier (BBB) can not only prevent harmful substances from invading the brain, but also shut out drugs1. As the main challenge of chemotherapeutic drug delivery, how to cross the BBB efficiently and safely is the key point of nanomaterials targeted therapy strategy2, 3. Glioblastoma (GBM) is one of the most common and invasive brain tumors, and even with the most effective surgical treatment, the median overall survival (OS) of patients with GBM remains less than 15 months4, 5. In addition, due to the characteristics of heterogeneity and invasiveness of GBM, it is difficult to completely resect the tumor tissues, thus making GBM be prone to recurrence and failing to be cured6. In order to achieve preoperative diagnosis and intraoperative positioning, there is an urgent need for the investigation of a relatively safe neurotumor targeted imaging probes. Accordingly, in the current study, we constructed a multifunctional therapeutic nanoplatform by integrating Car, ICG and magnetic nanoparticles, together with angiopep-2 modification for achieving NIRF/MR bimodal imaging and therapy for GBM.Methods and materials
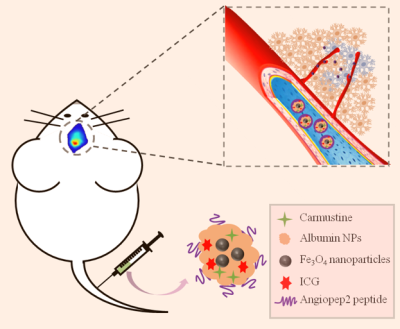
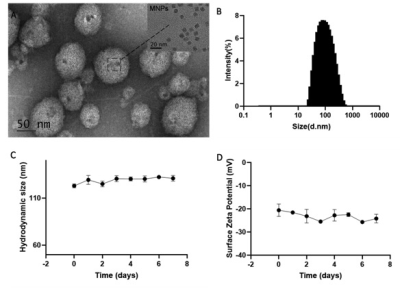
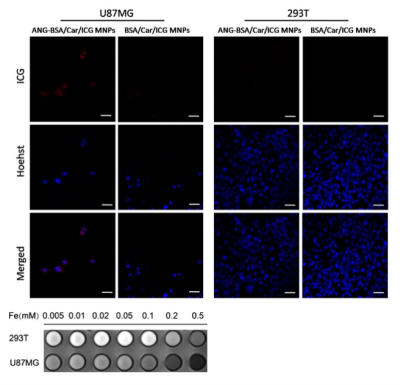
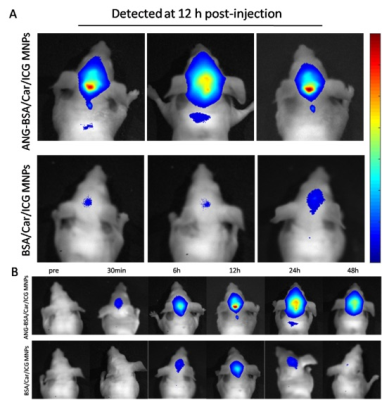
We constructed bovine serum albumin nanoparticles (ANG-BSA/Car/ICG MNPs) containing SPIO, carmustine and ICG coupled ANG (Figure 1). The in vitro and in vivo MRI/FL dual mode imaging of GBM were systematically evaluated in order to reflect the penetration ability of blood-brain barrier and tumor-specific targeting effect. At the same time, through experiments with the control group, we also evaluated the ability of ANG-BSA/Car/ICG MNPs to inhibit tumor.Results
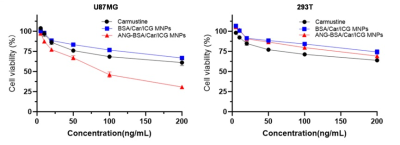
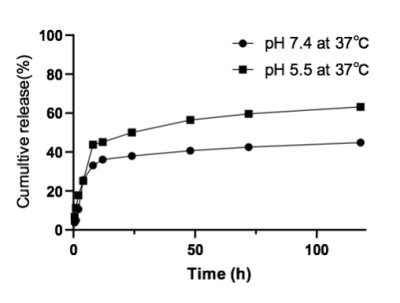
In vitro experiments, we verified that the targeting ability of nanoprobes to glioblastoma cells was significantly better than the control group (Figure 2-5). In addition, in vivo experiments, nanoprobes significantly increased the accumulation of brain tumors compared with the control group (Figure 6). This targeting imaging and drug delivery system provides an efficient strategy for targeted therapy and intraoperative localization of glioblastoma.Conclusion
ANG-BSA/Car/ICG MNPs present a promising potential in multifunctional therapeutic of glioblastoma, and we anticipate that this nanoplatform will be a perfect candidate in glioblastoma theranostic.Acknowledgements
This study is supported by a grant from Basic Plan Program of Shenzhen, China (No. JCYJ20180228163333734).
References
- Agarwal, S., et al., Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med, 2011. 13: p. e17.
- Liu, Y. and W. Lu, Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv, 2012. 9(6): p. 671-86.
- Osuka, S. and E.G. Van Meir, Cancer therapy: Neutrophils traffic in cancer nanodrugs. Nat Nanotechnol, 2017. 12(7): p. 616-618.
- Wen, P.Y. and S. Kesari, Malignant gliomas in adults. N Engl J Med, 2008. 359(5): p. 492-507.
- Krex, D., et al., Long-term survival with glioblastoma multiforme. Brain, 2007. 130(Pt 10): p. 2596-606.
- Huse, J.T. and E.C. Holland, Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer, 2010. 10(5): p. 319-31.
Figures