3045
Sub-5 nm Ultrafine Iron Oxide Nanoparticles for T1-Weighted MRI of Brain Tumors in Intracranial Mouse Model of Glioma1Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
Sub-5 nm ultrafine iron oxide nanoparticles (uIONP) is capable of entering intracranial U87 brain tumors in mice, leading to T1-contrast enhancement in the tumor similar to the clinically used gadolinium chelate contrast agents. The intra-tumoral accumulation of uIONP was supported by in vivo and ex vivo NIR imaging of NIR830-labeled uIONP and histological analysis. The developed uIONPs demonstrated faster clearance via kidney and liver comparing to conventional IONPs with larger sizes. Further development of uIONP-based MRI probes and drug carriers can provide a theranostic platform for precision medicine in brain tumors.
Introduction
Glioma remains one of the most fatal cancer types due to the lack of effective delivery of therapeutics as well as high recurrence caused by infiltrated cancer cells in normal brain tissue after surgical resection of tumor burden1. Although the continuous nonfenestrated blood vessels in the central nervous system (CNS), termed blood-brain barrier (BBB), have shown to compromise their functions in regulating CNS homeostasis and protecting from exogenous species in brains with tumors2, the effective systemic delivery of therapeutics to brain tumor remains challenging1. Hence, a theranostic platform capable of penetrating into tumors in the brain is urgently needed to provide precision medicine for imaging and treating gliomas. Iron oxide nanoparticles (IONPs) have demonstrated the capabilities as carriers for therapeutics and T2-weighted contrast agents for magnetic resonance imaging (MRI) by offering superb contrast enhancement, prolonged blood circulation and tumor retention time and facile surface functionalization3. However, the application of theranostic IONPs in brain tumors has been limited by their inefficient penetration through the BBB. Here, we report a sub-5 nm ultrafine IONP (uIONP) that is capable of entering and accumulating in intracranial glioma in mice. Unlike conventional IONPs demonstrating “darkening” T2 contrast enhancement, the developed uIONP exhibited comparable “bright” T1 contrast enhancement to clinically used gadolinium agents. The developed uIONP also showed faster clearance via the kidney and liver than conventional IONPs, which have larger sizes and are degraded in the liver.Materials and Methods
Preparation of uIONP: Iron (III) oleate is prepared using ferric nitride and sodium oleate dissolved in a mixed solvent of distilled water, hexane and absolute ethanol, and then mixed with 1-octadecene. After evaporating hexane, the reaction mixture is heated to 320 °C with a properly controlled heating rate. After cooling down to room temperature, ethanol is added to precipitate the hydrophobic uIONP, which is then dispersed in chloroform and added dropwise into the preheated glucose in dimethylformamide. The mixture was heated to form oligosaccharides on the IONP surface. After cooling down to room temperature, coated uIONPs are precipitated by adding ethanol, then washed repeatedly.Preparation of intracranial glioma mouse model: 8-Week old nude mice (Harlan Laboratories, Inc., Indianapolis, IN) were placed in a stereotaxic instrument, and 1 × 105 U87MG glioblastoma cells were inoculated into the right cortex using a procedure approved by the Emory University Institutional Animal Care and Use Committee. After 14 days, tumors were confirmed by Gd-DTPA enhanced T1-weighted MRI before use.
Magnetic resonance imaging (MRI) experiment: Tumor-bearing mice were scanned using a 3-Tesla scanner (Prizma, Siemens, Erlangen, Germany) using a standard head coil with mice placed in the iso-center of the magnet. MRI contrast and signal changes related to uIONPs were evaluated using T1- and T2-weighted fast SE sequence. The image analysis was carried out using the region of interest (ROI) method. After the mice were intravenously (i.v.) injected with uIONP (20 mg/kg) via tail vein, they were imaged at different time points.
Results and Discussion
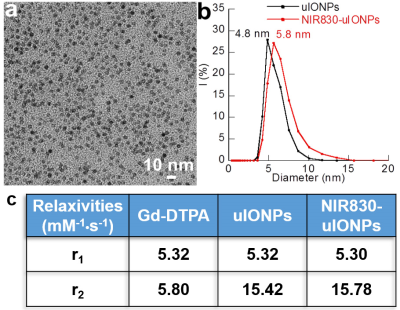
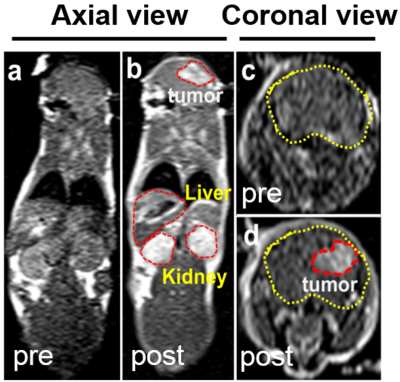
The developed uIONP displayed high uniformity and mono-dispersity in water as shown by the transmission electron microscopy (Fig 1a). The physio-chemical properties of uIONPs were further evaluated by dynamic light scattering (DLS) instrument to measure the hydrodynamic diameters and zeta potentials before and after the conjugation of near infrared (NIR) dye NIR830 (Fig 1b). The relaxivities of uIONP and NIR830-labeled uIONP were compared with clinically used Gd-DTPA T1 contrast agent (Fig 1c), revealing their similar capacity of generating T1-weight MRI contrast enhancement to Gd-DTPA.Comparing to pre-contrast images (Fig 2a and c), one-hour-post T1-weighted MRI of mouse received NIR830-labeled uIONP via i.v. administration showed contrast enhancement in the brain tumor (Fig 2b and d), which is similar to a typical Gd-enhanced T1-weighted MRI. However, unlike fast cleared Gd-DTPA, sustained T1 contrast induced by uIONPs over time indicated their long blood circulation time, which is critical for increased intra-tumoral accumulation of uIONPs via the exerted enhanced permeability and retention (EPR) effect4. Moreover, the “bright” T1 contrast was also observed in the highly vascularized organs, e.g. liver and kidney, (Fig 2b), reflecting the high blood stability and dispersity of NIR830-uIONPs. The accumulation of uIONPs in kidney also indicated the possibility of renal clearance of uIONPs, which may mitigate the concerns over long-term accumulation of uIONPs in liver as shown in larger sized conventional IONPs. Immediately after MRI, the ex vivo NIR imaging confirmed the MRI results with the strong NIR signals in liver, kidney and tumor located in the right cortex (Fig 3a and b). The confocal fluorescence microscopy of tumor section revealed NIR830-uIONPs escaping from the compromised BBB and penetrating into tumor regions (Fig 3c), further suggesting their increased tumoral accumulation with deep penetration and close interactions with tumor cells.
Conclusion
Sub-5 nm uIONP enhanced intracranial brain tumors with T1-weighted MRI contrast similar to the clinically used gadolinium chelate contrast agents. The intra-tumoral accumulation of NIR830-labeled uIONP was supported by in vivo and ex vivo NIR imaging and histological analysis. The developed uIONPs demonstrated faster clearance via kidney comparing to conventional IONPs which are degraded in liver.Acknowledgements
No acknowledgement found.References
[1] Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018; 20(2):184-191.
[2] Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015; 7(1):a020412.
[3] Huang J, Li Y, Orza A, et al. Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image‐guided approaches. Adv Funct Mater. 2016; 26(22):3818-3836.
[4] Wang L, Huang J, Chen H, et al. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1–T2 switchable magnetic resonance imaging contrast. ACS Nano 2017, 11 (5):4582-4592.
Figures