3044
A novel RXDLXXL-coupled peptide-conjugated USPIO nanoparticles for integrin αvβ6-targeted MR molecular imaging1Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, 2GE Healthcare, Beijing, China
Synopsis
Integrin αvβ6 is considered a promising molecular imaging target because it is significantly up-regulated in various types of cancers and correlates with the survival time of patients. MR imaging has advantages of non-invasive, non-radioactive, high soft-tissue contrast, and multiparameter imaging. Therefore, this study synthesized an integrin αvβ6-targeted nanoparticle probe for MR molecular imaging. Our experiments showed that novel targeted nanoparticles can specifically bind with integrin αvβ6 overexpressed cells in vitro and in vivo, presenting potential applications in the fields of αvβ6-positive tumor targeted MR molecular imaging.
Introduction
The motif RXDLXXL-based nanoprobes allow specific imaging of integrin αvβ6, a protein overexpressed during tumorigenesis and tumor progression of various tumors1, 2. Our study applied a novel RXDLXXL-coupled peptide conjugated with ultrasmall superparamagnetic iron oxide (USPIO) (referred to as cFK-9-USPIO) for the realization of integrin αvβ6-targeted MR molecular imaging.Materials and Methods
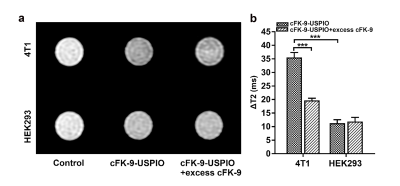
N-amino(-NH2)-modified ultrasmall superparamagnetic iron oxide nanoparticles (USPIO) were conjugated with integrin αvβ6-targeted peptide cFK-9 by dehydration esterification reaction to create a novel MR targeted nanoparticle probe cFK-9-USPIO. The synthesis route of targeted probe is shown Figure1a.Integrin αvβ6-positive mouse breast cancer cell lines (4T1) and integrin αvβ6-negative human embryonic kidney cell lines (HEK293) were incubated respectively with cFK-9-AbFlour 647 and cFK-9-USPIO (targeting group), and subsequently imaged using laser scanning confocal microscopy (LSCM) and 3.0 Tesla MRI system (Discovery MR 750; GE Healthcare, Milwaukee WI, USA). The mean fluorescent intensity was applied to semi-quantitatively analyze affinity of cFK-9 targeting αvβ6 and changes of T2 values (ΔT2) were calculated using T2-multi-echos pulse sequence (TR=1700ms, TE=9.4-75.2ms (8TE), FOV=80mm×64mm, data matrix=128×128, slice thickness=3mm, spacing=0.3mm) to evaluate the targeting effect of MR imaging in vitro.
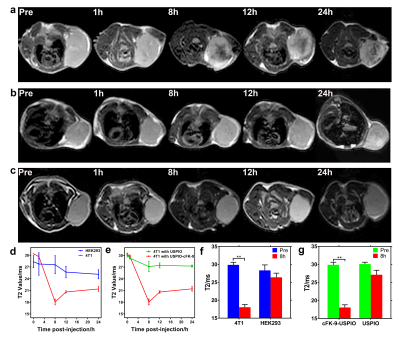
The in vivo MR imaging capability of cFK-9-USPIO was investigated in the 4T1 xenograft models. T1- and T2-weighted imaging were performed: TR of 400ms and TE of 10.3ms were used for T1WI and TR of 1500ms and TE of 88.8ms were used for T2-weighted fast spin echo imaging, a FOV of 6mm×6mm, slice thickness of 1mm with 0.8mm spacing. The imaging sequences of the multi-T2 map were described as follows: TR/TE 1500ms/7.2-57.3ms (8TE), slice thickness 1mm with spacing 0.8mm. T2 values of each tumor in mice were obtained from T2map at GE Advantage Workstation 4.6.
Tumors and normal tissues of the liver, spleen, kidney and muscle were collected and these samples were fixed in formaldehyde solution at 4℃ for 24h prior to embedding in paraffin wax. Tissue sections were stained with Hematoxylin and eosin (H&E) or Prussian blue solution and observed under a light microscope.
SPSS 24.0 (SPSS Inc., Chicago, USA) were applied for data management and statistical analysis. Data are expressed as mean±standard deviation (SD) and analyzed by independent samples t-test and one-way analysis of variance (ANOVA), where p values below 0.05 were considered as statistically significant.
Results
Figure1 shows the characteristics of the targeted magnetic probe. The synthesis route of targeted nanoparticles was shown in Figure1a. The core sizes of cFK-9-USPIO determined by TEM was 5.52±0.81nm (Figure1b) and cFK-9-USPIO nanoparticles had a short T2 relaxation time (Figure1c) with an r2 value of 122.8mM-1S-1 (Figure1d). The difference of fluorescent intensity between targeting group and blocking group in 4T1 was significantly greater than in HEK293 cells (P﹤0.01) in LCSM imaging. In vitro MR imaging showed a more remarkable T2 reduction in 4T1 cell lines than HEK293 cell lines (p﹤0.01) (Figure2). Similar results are also observed in the 4T1 tumor-bearing nude mice (Figure3), and subsequent histological examination proved the specific accumulation of targeted nanoparticles in 4T1 integrin αvβ6-positive tumors.Discussion and Conclusion
Recent studies have developed various radioactive molecular probes for PET or SPECT imaging of integrin αvβ6 expression in preclinical animal models, yet few reports research integrin αvβ6-targeted MR molecular imaging3, 4. In view of the advantage that MRI is non-invasive, non-radioactive and provides multiparameter imaging with high soft-tissue contrast, we designed novel integrin αvβ6-targeted nanoparticles and verified that these nanoparticles specifically bound with integrin αvβ6-positive tumor cells in vitro and selectively accumulated in xenograft tumors in vivo MRI approaches. Our findings showed that integrin αvβ6-targeted nanoparticles have great potential to the detection of αvβ6-positive cancer with MR molecular imaging.Acknowledgements
This study was supported by the National Natural Science Foundation of China grants (no.81671757).References
1. Niu J, Li Z. The roles of integrin αvβ6 in cancer. Cancer letters. 2017; 403:128-37.
2. Maltsev OV, Marelli UK, Kapp TG, et al. Stable Peptides Instead of Stapled Peptides: Highly Potent αvβ6-Selective Integrin Ligands. Angewandte Chemie (International ed in English). 2016; 55(4):1535-9.
3. Liu H, Wu Y, Wang F, et al. Molecular imaging of integrin αvβ6 expression in living subjects. American journal of nuclear medicine and molecular imaging. 2014; 4(4):333-45.
4. Notni J, Reich D, Maltsev OV, et al. In Vivo PET Imaging of the Cancer Integrin αvβ6 Using 68Ga-Labeled Cyclic RGD Nonapeptides. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017; 58(4):671-7.
Figures
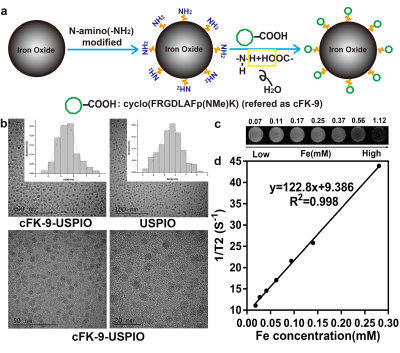
Figure 1. Characterization of the nanoprobe. (a)The schematic synthesis route of cFK-9-USPIO. (b) The size and histogram analysis of cFK-9-USPIO under transmission electron microscope (TEM). (c) T2-Weighted MRI images (3.0T, Discovery MR 750, GE Medical System, Milwaukee WI) of increasing cFK-9-USPIO concentration. (d) T2-shortening effect of cFK-9-USPIO nanoparticles at 3.0T.