3042
In vivo evaluation of metabolic response to dobutamine stimulation in ischemic rat hearts using hyperpolarized 13C-pyruvate1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 3Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 4Chemistry, The University of Texas at Dallas, Richardson, TX, United States
Synopsis
Heart failure due to ischemic coronary artery disease is a leading cause of morbidity and mortality. Metabolic assessment of myocardium could be important for assessing myocardial ischemia in patients. This study evaluated in vivo metabolism of hyperpolarized 13C-pyruvate in ischemia rat hearts under normal and elevated workload by dobutamine stimulation. Decreased production of bicarbonate was observed in hearts with ischemic myocardium compared to control hearts. Dobutamine stimulation lowered bicarbonate production in control hearts but did not change the appearance of bicarbonate signals in ischemic group.
INTRODUCTION
Ischemic heart failure is a leading cause of morbidity and mortality.1 Multiple imaging techniques have been evaluated for detection of ischemic myocardium (IM) in various models including the stenosed coronary artery model.2 Conventional methods such as PET, dobutamine stress echo or late gadolinium enhancement do not directly detect the functional state of the key organelle in oxidative metabolism, the mitochondrion. Hyperpolarized (HP) 13C-metabolic imaging has been used to assess mitochondrial function in preclinical models3 and is a potential modality for evaluating ischemic myocardium noninvasively. The major goal of this study is to evaluate myocardial response to dobutamine stimulation in ischemic rat hearts using HP-[1-13C]pyruvate.METHODS
Study groups and animals: Animal studies were approved by IACUC. Metabolism of HP [1-13C]pyruvate was investigated in male SD rats in four groups: 1. normal myocardium (NM), 2. ischemic myocardium (IM), 3. NM+dobutamine, 4. IM+dobutamine. To induce myocardial ischemia, rats were anesthetized by intraperitoneal injection of ketamine/xylazine (50/2.5 mg/kg). A left parasternal thoracotomy was performed in the 4th–5th intercostal space following incubation and ventilation with 1% isoflurane mixed with oxygen. The left atrium was elevated to expose the left coronary artery and snare was positioned around the vessel in contact with a 300 μm diameter probe 1–2 mm from its origin. The vessel was allowed to expand upon probe removal, resulting in a partial occlusion of the coronary artery, a model described previously.4 For dobutamine stress HP-MRS, an intra-peritoneal injection of 1.5 mg/kg of dobutamine was administered before positioning the animal in the MRI scanner. 13C MRS and 13C chemical shift imaging (13C-CSI) of these animals were carried out 7 days after the surgery.Polarization and injection of [1-13C]pyruvate: Neat [1-13C]pyruvic acid was mixed with 15 mM trityl radical OX63, and Gadoteridol ([Gd3+]=2 mM) was polarized at ~1.4 K, 3.35 T for 1.5 h in a HyperSense® polarizer. The polarized substrate was quickly dissolved in 4 mL hot PBS buffer (pH 7.4) and neutralized with NaOH. Hyperpolarized pyruvate solution (80 mM) was injected via a cannulated tail vein catheter over 12-15 seconds.
In-vivo 13C-MRS and 13C-CSI: 13C data were acquired in a 4.7 T MRI scanner (Agilent, Palo Alto, CA) using a Varian 60 mm dual tuned 1H/13C linear volume coil. Anatomical 1H images (GEMS; matrix size: 128x128; FOV: 60x60 mm; slice thickness: 2 mm) were acquired for slice planning and positioning. 13C intensity maps were generated and displayed as overlays on grayscale 1H anatomical images. In separate experiments, in vivo HP-13C-MRS data acquisition (flip angle: 10 deg, TR: 2s, sw: 15000 Hz) was initiated immediately after the pyruvate injection was completed. HP-13C-MRS data were analyzed by measuring the area-under-the-curve (AUC) for the lactate, alanine and bicarbonate signals normalized to the total 13C signal from lactate, alanine and bicarbonate. Plots of bicarb/lactate, bicarb/alanine and lactate/alanine were generated from these data. HP-13C-CSI (8x8, TE:1.4 ms, TR:36.7 ms, sw:7485 Hz, data points: 512, thickness:12mm) data was also acquired to evaluate the spatial distribution of the metabolites in the myocardium. Statistical significance was evaluated by unpaired Student’s t-test using GraphPad Prism (v.8).
RESULTS AND DISCUSSION
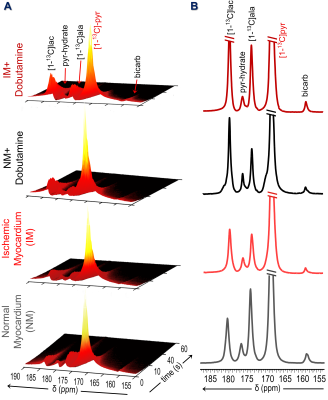
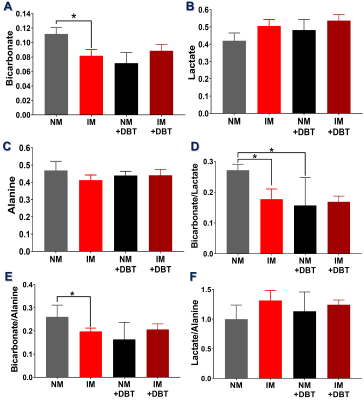
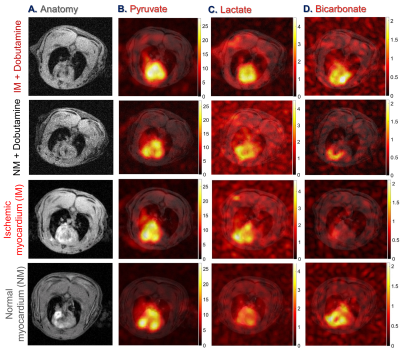
Representative sequential 13C-MR spectra (Fig. 1A) acquired from an 18-mm axial slice of the heart show the evolution of 13C-metabolites after injection of HP-[1-13C]pyruvate. The corresponding summed 13C spectra (Fig. 1B) show well-resolved signals from [1-13C]lactate, [1-13C]alanine, and 13C-bicarbonate. In hearts with partial coronary ligation, the spectroscopy data were acquired from a mixture of ischemic and nonischemic myocardium. A 27% decrease in bicarbonate production was observed in hearts with ischemic myocardium compared to control hearts (Fig. 2A). After stimulation of NM with dobutamine, less bicarbonate was produced from HP-pyruvate likely due to an increased contribution of other endogenous substrates (circulating fatty acids/glucose/ketones) to acetyl-CoA. Production of bicarbonate was unchanged in IM hearts following adrenergic stimulation. Production of HP-lactate (Fig. 2B) from HP-pyruvate trended toward higher levels in both the IM and after dobutamine stimulation but these differences did not reach statistical significance. Alanine production (Fig. 2C) was comparable in both groups, with or without dobutamine, with high variability in signal intensity (Fig. 1). The average bicarbonate to lactate ratio (Fig. 2D) in IM hearts is 37% lower than the ratio in NM hearts. A 44% decrease in the bicarbonate-to-lactate ratio was observed as a result of dobutamine stimulation of NM hearts. Dobutamine did not change this ratio in IM hearts. A similar trend was observed when comparing bicarbonate-to-alanine ratios across all four groups of hearts (Fig. 2E). The lactate-to-alanine ratios (Fig. 2F) tended to be higher in both NM and IM hearts after dobutamine stimulation but, again, these differences did not reach statistical significance. Figure 3 show representative intensity maps of 13C-bicarbonate, 13C-lactate, as well as the injected substrate 13C-pyruvate overlayed onto anatomical 1H images. These images confirm that pyruvate metabolism as detected by the in vivo 13C-spectroscopy largely detects the myocardium. The trend of the metabolic intensity maps agrees well with the quantitative analyses of 13C-pyruvate metabolism shown in Fig. 2.CONCLUSION
Taken together, our results demonstrate that decreased oxidative metabolism of pyruvate is observed as a result of myocardial ischemia and dobutamine stimulation. No change in bicarbonate appearance was observed ischemic hearts even after adrenergic stimulation.Acknowledgements
Funding support: AHA 18POST34050049, NIH 5R37-HL034557, and NIH P41-EB015908.References
1. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL and Trogdon JG. Forecasting the Impact of Heart Failure in the United States. Circulation: Heart Failure. 2013;6:606-619.
2. Kramer CM, Sinusas AJ, Sosnovik DE, et al. Multimodality imaging of myocardial injury and remodeling. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51 Suppl 1(Suppl 1):107S-121S.
3. Apps A, Lau J, Peterzan M, Neubauer S, Tyler D and Rider O. Hyperpolarised magnetic resonance for in vivo real-time metabolic imaging. Heart. 2018;104:1484-1491.
4. Capasso JM, Jeanty MW, Palackal T, Olivetti G, Anversa P. Ventricular remodeling induced by acute nonocclusive constriction of coronary artery in rats. Am J Physiol 1989;257:H1983–93.
Figures