3039
Progression towards in-vivo detection of Signal Amplification by Reversible Exchange (SABRE) Hyperpolarisation
Aneurin J Kennerley1, Marie-Christine BD Labarthe1, Elizabeth J Fear1, Peter J Rayner1, and Simon B Duckett1
1Chemistry, University of York, York, United Kingdom
1Chemistry, University of York, York, United Kingdom
Synopsis
We demonstrate that signal amplification by reversible exchange (SABRE) presents as a promising and fast route to hyperpolarising a wide range of biologically relevant contrast agents, including nicotinamide (a precursor for NAD+ a mediator of metabolism) and pyruvate (for mapping anaerobic metabolism) for in-vivo preclinical magnetic resonance imaging and spectroscopic detection. Images recorded in a rat model at 7 Tesla (Bruker BioSpec 70/30) are presented. We highlight necessary chemical preparation steps to ensure that the SABRE agents are bio-compatible following exposure to high pressure para-hydrogen gas and the heavy metal Iridium catalyst required for SABRE hyperpolarisation.
Introduction
Signal amplification by reversible exchange (SABRE) is a promising route to hyperpolarising a wide range of biologically relevant contrast agents for magnetic resonance1,2. SABRE utilises singlet state para-hydrogen (p-H2) as the source of polarisation3. Spin polarisation transfers to the target agent via a catalytic and reversible exchange (Figure 1) and importantly does not change the chemical identity of the agent/substrate it hyperpolarizes. Additionally the SABRE process takes just a few seconds and does not require expensive hardware to achieve (like other polarisation methods). SABRE polarization transfer between p-H2 and the agent occurs through concurrent binding to an iridium catalyst in a solvent such as deuterated alcohols. Up to 50% 1H polarisation for di‐deuterio‐nicotinate using this method have been reported4. However such solvents limit the biocompatibility for in-vivo investigation of SABRE hyperpolarised agents. Here we present recent progress in the development of bio-compatible routes to SABRE hyperpolarisation5. This method relies on a biphasic solvent system to separate the hyperpolarised agent into a D2O layer for subsequent safe injection. As such we have now captured 1H and 13C in-vivo preclinical MR images of this novel hyperpolarisation technology; working towards fast and efficient mapping of real time in-vivo biochemistry for application in cardiology, neurology and oncology.Methods
All aspects of these methods and their development were performed with UK Home Office approval under the Animals (Scientific Procedures) Act 1986. Female Sprague-Dawley rats (n=12) weighing (250–400 g) were kept in a 12 h dark/light cycle, at a constant temperature of 22 °C, with food and water ad libitum. Prior to surgery, animals were anesthetised with urethane (1.25 g/kg i.p). Rectal temperature was maintained at 37 °C throughout surgical and experimental procedures using a homeothermic blanket (Harvard Apparatus Inc, USA). Animals were artificially ventilated and both left/right hand side femoral arteries and veins cannulated for invasive monitoring of arterial BP/blood gases and intravenous infusion respectively. Phenylephrine was infused to increase BP to normal physiological limits with blood gas measurements taken periodically (pO2 = 105 mm Hg ± 4; pCO2 = 38 mm Hg ± 5).Following surgery animals were inserted into the bore of a 7 Tesla pre-clinical MRI scanner (Bruker BioSpec 70/30, 310mm bore with preinstalled BGA-12s imaging gradients). An 86mm ID volume coil (Quadrature 1H or dual tuned 1H/13C for 1H or X-nuclei detection) was used for RF TX/RX. A 1.5 mL sub-cutaneous or 0.4 mL intravenous bolus injection of SABRE hyperpolarised agents (nicotinamide, methyl-nicotinate, thienopyridazine and pyruvate) were administered. These agents were chosen for both ease of SABRE hyperpolarisation (fringe field polarisation transfer fields) and their biochemical/medical importance. Nicotinamide is a precursor for NAD+, an important mediator for cellular metabolism. Methyl-nicotinate is a rubefacient with known vaso-dilatory effects. The thienopyridazine motif has anti-cancer properties, inhibiting IKK6. The use of hyperpolarised pyruvate has already been proven (with dynamic nuclear polarisation) as good marker for detecting anaerobic metabolism in cancer7.
Subsequent MR measurements utilised either standard (RARE, FISP & FLASH) or spectroscopic (CSI & EPSI) imaging pulse sequences for 1H and 13C nuclei. Functional images were superimposed on high resolution structural scans where appropriate. 1H imaging was also tested with long duration saturation RF pulses and spectra-spatial RF pulses to null contamination effects from the abundant proton water pool.
Results
We showcase in-vivo 1H SABRE hyperpolarised subcutaneous images of nicotinamide, methyl-nicotinate (figure 2), thienopyridazine, and 13C images of pyruvate. We show that EPSI produces robust spectral responses across all 1H agents, with T1 times of ~40s (sequence repetition time ~6s) and reduced under-sampling/alias artefacts when compared to low spatial resolution FID based CSI (sequence repetition time ~1.2mins). However, EPSI measurements require substantial post processing to limit water contamination and ghosting artefacts from reversed echoes off resonance. Data are presented which show that significant volumes of hydrogen remain dissolved, even in water based bio-compatible solvents. We therefore add a sonication step before infusion;however polarisation levels decrease substantially (100 fold - figure 2) during this process. Residual heavy metal catalyst is sequestered from the solution (confirmed with inductively coupled plasma mass spectrometry). I.V injection of SABRE hyperpolarised nicotinamide, following these post polarisation procedures, is shown to have no adverse effects on animal physiology. In separate experiments cardiac, brain and tumour based detection was undertaken.Conclusion & Discussion
This research demonstrates the recent advances in the in-vivo detection of SABRE hyperpolarised agents; detailing developments to help realise its potential as a valuable weapon for in-vivo metabolic monitoring. We have generated hyperpolarised images for biochemical/medically important nicotinamide, methyl-nicotinate, thienopyridazine and pyruvate. Imaging utilises both 1H and X-nuclei imaging strategies. Targeted biochemical functionalisation of these agents to map in-vivo metabolism through spectral differentiation is on-going.Acknowledgements
Financial support from the Wellcome Trust (Grants 092506 and 098335) and the MRC (MR/M008991/1) is gratefully acknowledged by the University of York. We thank the technical staff of the biological services facility (H Daniels & C Turnbull) and chemistry department (Dr R John and Dr V Annis) for their assistance.References
- Adams, R.W., Aguilar, J.A., Atkinson, K.D., Cowley, M.J., Elliott, P.I., Duckett, S.B., Green, G.G., Khazal, I.G., Lopez-Serrano, J., Williamson, D.C. (2009) Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science, 323, 1708.
- Duckett, S.B., Lali, W., Roy, S.S., Tickner, B.J., Ahwal, F., Kennerley A.J., (2019) Hyperpolarizing Pyruvate via Signal Amplification By Reversible Exchange (SABRE). Angewandte Chemie 131(30) 10377-81
- Bowers, C.R. & Weitekamp, D.P. (1986) Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 57, 2645–2648.
- Rayner, P.J. & Duckett, S.B. (2018) Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angewandte Chemie 57(23) 6742-53.
- Lali, W., Olaru, A.M., Green, G.G., Duckett, S.B. (2017) Achieving High Levels of NMR‐Hyperpolarization in Aqueous Media With Minimal Catalyst Contamination Using SABRE. Chem. Eur. J., 23 (44), 10491-10495;
- Hoffman-La Roche, A.G., Labadie, S.S., Sjogren, E.B., Talamas, F.X. (2005) Thienopyridazines as IKK inhibitors. World Intellectual Property Organisation Patent No. WO 2005/105606
- Gutte, H., Hansen, A.E., Johannesen, H.H., Clemmensen, A.E., Larsen, J.H.A., Nielsen, C.H., Kjaer, A. (2015) The use of dynamic nuclear polarization 13C-pyruvate MRS in cancer. Am J Nucl Med Mol Imaging. 5(5) 548-60
Figures
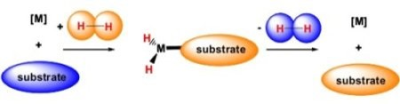
Schematic
of SABRE hyperpolarisation process. Spin polarisation transfers from para-hydrogen to the target agent by reversible exchange across an Iridium based catalyst. The process is fast (~10s) and importantly does not change the chemical identity of the agent/substrate it
hyperpolarizes.
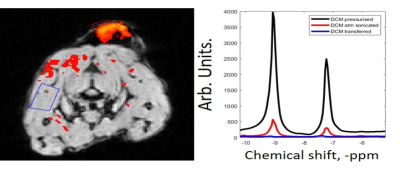
In-vivo sub-cutaneous imaging of SABRE hyperpolarised
methyl-nicotinate using an EPSI sequence. Spectral peaks at ~9.1 and 7.3ppm are measured
with polarisation levels assessed across different steps of the agent
preparation.