3038
Towards Comprehensive Assessment of Lung Injury Using Multi-Modality Hyperpolarized MRI
Mehrdad Pourfathi1, Hooman Hamedani1,2, Yi Xin1,2, Stephen J Kadlecek1, Ian Duncan1, Maurizio Cereda1,3, Sarmad Siddiqui1, Harrilla Profka1, Luis Loza1, Faraz Amzajerdian 1,2, Tahmina Achekzai1, Kai Ruppert1, Michael Rosalino1, Federico Sertic1,4, Ryan Baron1, Jon Snow1, Yiwen Qian1,2, Gabriel Unger1, Shampa Chatterjee5, and Rahim R. Rizi1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 4Surgery, University of Pennsylvania, Philadelphia, PA, United States, 5Physiology, University of Pennsylvania, Philadelphia, PA, United States
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 4Surgery, University of Pennsylvania, Philadelphia, PA, United States, 5Physiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Hyperpolarized 13C and 129Xe MRI are novel imaging methods that have been used to characterize tissue metabolism and lung function, respectively. In this study, we demonstrate the use of these techniques in tandem in order to characterize lung injury in a porcine model of aspiration pneumonitis.
Introduction
Chest radiography and computed tomography (CT) are used to monitor the progression or resolution of acute lung injury. While powerful, these techniques only reveal structural and morphological changes in the tissue, which may supersede otherwise undetectable functional and cellular changes. Recent advancements in hyperpolarized (HP) MRI technologies have enabled new approaches to probe regional structural and functional parameters in the lungs using 129Xe MRI and lung tissue metabolism using [1-13C] pyruvate MRI1,2. Here, we sought to demonstrate the combined use of these technologies to assess lung injury in pig model of aspiration pneumonitis, using a setup similar to what would be used clinically. Our data shows the potential for an such integrated imaging approach to comprehensively assess lung injury.Materials and Methods
Two Yorkshire pigs were ventilated (VT=10 ml/kg, PEEP=0-5 cmH2O, FiO2=0.5-1, f=15 min-1) in supine position. Hydrochloric acid (HCl, pH 1.0) was delivered to the base of the lungs using a bronchoscope, after which animals remained on the ventilator. Animals’ temperature was kept between 36.5-38.5°C during the study using a blanket that circulates heated water. 7 hours after acid instillation, 129Xe imaging was performed using a 1.5T Siemens Avanto clinical MRI system. 129Xe signal was acquired using an 8-channel 129Xe flex-coil. 2L of enriched 129Xe gas was polarized using a XeBox-E10 commercial prototype polarizer (Xemed, LLC, Durham, NH). A mixture of 20% oxygen and 80% HP 129Xe was delivered to the pigs using a custom-made ventilator. Two-dimensional projection gas and dissolved phase axial images were simultaneously obtained using an RF-spoiled GRE sequence (in-plane FOV = 20x57 cm2, matrix-size = 28x80, TR/TE = 30/2.4 ms, flip-angle (dissolved/gas) = 20o/1o with a 900 ms Gaussian pulse centered on the dissolved phase peak)3. After a 1-hour 129Xe imaging session, animals were transported to a 3T Siemens Trio clinical MRI system for 13C imaging; 13C images were obtained using an 8-channel 13C flex-coil. Samples containing 640 mg of [1-13C] pyruvate mixed with 15mM of AH111501 EPA were polarized using a 5T SpinLab DNP hyperpolarizer. 1ml/kg of 250 mM neutralized isotonic HP [1-13C] pyruvate sample was injected through the ear vein over 30 seconds, and chemical shift imaging was started using a modified FID-CSI sequenced 40 seconds after the start of injection (in-plane FOV=20x20 mm2, slice-thickness=5 cm, matrix-size=16x16, flip-angle=6o, spectral-width=2,560 Hz, TR=52 ms). A 15 second breath-hold was applied to minimize imaging artifacts due to respiratory motion. Localizers and T2-weighted anatomical images were obtained prior to each HP study.Results and Discussion
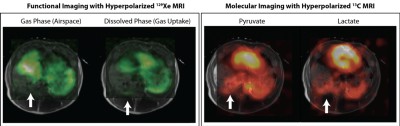
Representative HP 129Xe gas and dissolved phase maps as well as HP [1-13C] pyruvate and [1-13C] lactate maps overlaid on the corresponding anatomical T2 image are shown below. Lactate signal is elevated in the posterior region of both lungs; however, the 129Xe scan shows low ventilation and gas uptake only in the right lung (white arrow). We previously showed that HP 13C MRI is capable of detecting regions with elevated lactate production in the lungs due to injury in-vivo2. Although our findings suggested that elevated lactate-to-pyruvate ratio is strongly associated with early inflammatory activity, it is not unambiguously identifiable as a precursor to lung injury progression since it may also result from hypoxia, ischemia or atelectrauma. Using HP 129Xe MRI, we can identify injury-induced structural and functional alterations by obtaining local measurements of oxygen tension, specific ventilation and gas uptake3,4. This additional information can potentially improve the specificity of our 13C HP-MRI: e.g., by co-localizing the alveolar oxygen tension map attained with HP 129Xe MRI and the lactate-to-pyruvate map measured via 13C HP-MRI, allowing us to sensitively distinguish between hypoxia and inflammatory response.Conclusions
In this study, we demonstrated the feasibility of using HP 13C and 129Xe imaging as a multi-modality approach to characterize lung injury. While 13C MRI highlights regions with abnormal metabolism, 129Xe MRI can provide additional information regarding gas uptake and ventilation, thereby enabling a more comprehensive characterization of the injured lung. This can ultimately lead to better patient outcomes by enabling both earlier injury detection and earlier selection of the appropriate management strategy to resolve the injury.Acknowledgements
This work was supported by NIH (Bethesda, MD, USA) grants R01-HL124986 and R01-HL139066.References
1. Ruppert, K. et al., Sci. Rep. 9, 1–11 (2019).
2. Pourfathi, M., nature.com 8, 280 (2018).
3. Ruppert, K. et al. Sci. Rep. 8, 7310 (2018).
4. BA, T. A. et al. Acad. Radiol. 26, 367–382 (2019).