3037
Trehalose as an alternative to glycerol as a glassing agent for in vivo DNP MRI1Radiation Biology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, MD, United States, 2Department of Chemistry & Biochemistry, University of Denver, Denver, CO, United States
Synopsis
Glassing agents are essential in DNP studies but have the potential to perturb the metabolic measurements that are being studied. Glycerol, the glassing agent of choice for in vivo DNP studies, is effective at reducing ice crystal formation during freezing but is rapidly metabolized, potentially altering the redox and ATP balance of the system. As a biologically inert alternative to glycerol, we show here that 15-20 wt % trehalose yields a glass that polarizes samples more rapidly than the commonly used 60% wt formulation of glycerol and yields similar polarization levels within clinically relevant timeframes.
Purpose:
Glycerol has emerged as the glassing agent of choice for in vivo DNP studies, due to its favorable safety profile and relatively high glassing efficiency. Although glycerol is safe at the concentrations used in DNP studies, it is not metabolically inert. Glycerol is rapidly transported into the cell by aquaporin1 and readily metabolized to glycerol-3-phosphate in the liver,2 potentially perturbing metabolic measurements by the consumption of ATP and the reduction of FAD during the glycerol-3-phosphate shuttle. An improved glassing agent with a favorable safety profile that does not affect metabolism would help expand the clinical translation of DNP beyond pyruvate to other metabolic tracers. Trehalose is a non-reducing disaccharide commonly used as a pharmaceutical excipient to stabilize proteins in an immobile glass matrix.3 Trehalose is clinically approved for IV injections as high as 1 g/kg, far exceeding the range expected in dissolution DNP. In contrast to glycerol, trehalose is only metabolized in the small intestine and is therefore metabolically inert in IV injections.Methods:
DNP polarization experiments were performed on a 1.5 T Oxford Hypersense according to standard protocols using 15 mM Oxo63. Hyperpolarized 13C MRI studies were performed on a 3 T scanner (MRSolutions) using a 17 mm 13C solenoid coil inside a saddle coil for 1H imaging. Polarization buildup rates were calculated assuming single exponential growth.Electron spin relaxation times were measured at 5K using a Bruker E580 pulsed EPR spectrometer. T1 values were calculated from UPEN analysis4 of inversion recovery experiments with most measurements made near the peak of the field-dependent spectrum. Multiple choices of time windows were included in the relaxation measurements to include both the short and long relaxation times.
Results:
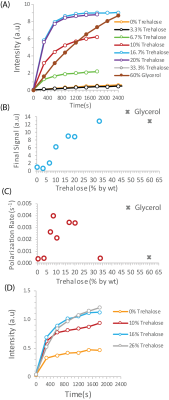
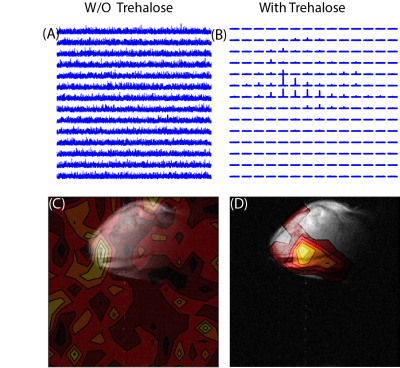
To test the potential of trehalose as a biocompatible glassing agent, we recorded the polarization buildup curves of 25 wt% 13C urea, a potential marker for perfusion,5 in the presence of increasing amounts of trehalose at 3.5 T. In the absence of trehalose, polarization of urea was slow and the buildup of polarization was negligible in clinically relevant timeframes (Figure 1A orange line). Imaging of a mouse leg xenograft with hyperpolarized urea without trehalose yielded only noise with no discernable signal (Figure 2A).The addition of trehalose markedly increased both the equilibrium polarization and buildup kinetics. Starting at 6.7%, the final signal increases rapidly with concentration before leveling off at approximately 15% trehalose. A similar concentration dependence is also seen with the buildup rate (Figure 1C). In comparison to the standard 60% glycerol preparation,6 trehalose solutions build up polarization much more quickly but have a ca. 25% lower equilibrium polarization relative to no glassing agent. The increase in polarization led to greatly enhanced imaging in vivo. While the urea image without a glassing agent was completely noise, the addition of 20 % trehalose in the polarization mixture results in a clear image with urea localized within the tumor (Figure 2B). To confirm the results are not confined to urea, we repeated the polarization experiments with another difficult to polarize, non-self glassing substrate, 13C labelled glycine, with similar results (Figure 1D).
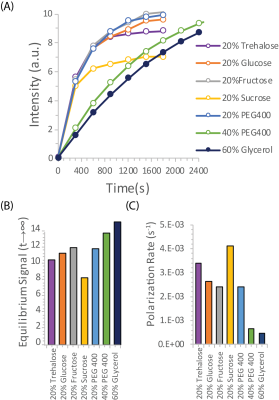
To evaluate other potential carbohydrate glassing agents besides trehalose, we measured the polarization buildup of urea in the presence of equivalent amounts of analogous mono- and disaccharides (Figure 3). Trehalose was not unique in this context; fructose, glucose, and PEG400 all had slightly higher equilibrium polarization at an equivalent wt %. Sucrose had significantly lower equilibrium polarization but slightly faster build up kinetics. In comparison, 20% trehalose was an effective compromise between fast buildup and equilibrium polarization, allowing efficient polarization within a clinically relevant timeframe.
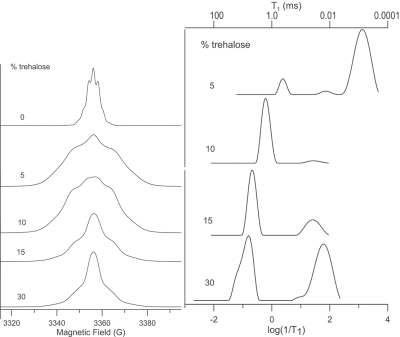
To gain a greater understanding of how trehalose may affect changes in the microenviroment of the Oxo63 radical during the DNP process, we recorded the EPR spectra and spin-lattice (T1) relaxation times of Oxo63 at 5K as a function of trehalose concentration (Figure 4). Changes in trehalose concentration resulted in dramatic changes in both the magnitude and distribution of relaxation times (Figure 4) of Oxo 63. At 5% trehalose, where DNP efficiency is similar to samples without trehalose (Figure 1), the T1 relaxation is dominated by a fast relaxing population with a very short relaxation time (ca ~0.8 ms) similar to that found in the trehalose-free sample (Figure 4). This is consistent with the water crystallizing upon freezing, causing high local concentrations of OX063 as the radical is preferentially excluded from ice crystals. Increasing concentrations of trehalose up to about 15% caused both a population shift towards the slower relaxing species and within the slower relaxing population, a shift towards slower relaxation times. The highest concentration,30%, partially reverses this trend as there is a large shift in the distribution back to short relaxation times. Overall, there is an approximate correspondence between the T1 relaxation times and DNP build up kinetics. Longer relaxation times are associated with faster build up kinetics, as expected for narrow linewidth radicals like Oxo63 where DNP transfer is dominated by the solid effect.
Conclusions:
Trehalose is an attractive alternative glassing agent for situations where there may be concerns about glycerol’s glucogenic potential and possible alteration of ATP/ADP and redox balance within the timescale of the DNP experiment.Acknowledgements
No acknowledgement found.References
[1] Hibuse, T., Maeda, N., Nagasawa, A., and Funahashi, T. (2006) Aquaporins and glycerol metabolism, Bba-Biomembranes 1758, 1004-1011.
[2] Tao, R. C., Kelley, R. E., Yoshimura, N. N., and Benjamin, F. (1983) Glycerol - Its Metabolism and Use as an Intravenous Energy-Source, Jpen-Parenter Enter 7, 479-488.
[3] Richards, A. B., Krakowka, S., Dexter, L. B., Schmid, H., Wolterbeek, A. P. M., Waalkens-Berendsen, D. H., Shigoyuki, A., and Kurimoto, M. (2002) Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies, Food Chem Toxicol 40, 871-898.
[4] Bortolotti, V., Brown, R. J. S., Fantazzini, P., Landi, G., and Zama, F. (2017) Uniform Penalty inversion of two-dimensional NMR relaxation data, Inverse Probl 33.
[5] von Morze, C., Larson, P. E., Hu, S., Keshari, K., Wilson, D. M., Ardenkjaer-Larsen, J. H., Goga, A., Bok, R., Kurhanewicz, J., and Vigneron, D. B. (2011) Imaging of blood flow using hyperpolarized [(13)C]urea in preclinical cancer models, J Magn Reson Imaging 33, 692-697.
[6] Wilson, D. M., Keshari, K. R., Larson, P. E. Z., Chen, A. P., Hu, S., Van Criekinge, M., Bok, R., Nelson, S. J., Macdonald, J. M., Vigneron, D. B., and Kurhanewicz, J. (2010) Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo, J Magn Reson 205, 141-147.
Figures