3035
Simultaneous 19F hyperpolarization of aromatic molecules in aqueous solution1Institute of Biometrics and Medical Informatics, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
Synopsis
Fluorinated aromatic substrates are of high interest in biomedical and pharmaceutical applications. To increase the low 19F MR signal intensity in aqueous solutions, the hyperpolarization technique photo-Chemical Induced Dynamic Nuclear Polarization (photo-CIDNP) can be used. Compounds such as 2-fluoro-tyrosine, 3-fluoro-tyrosine or 3-fluoro-4-hydroxyphenylacetic acid were hyperpolarized repeatable inside the detection field (7T). Strong 19F signal enhancements could be detected for aromatic systems, which were fluorinated in meta-position. The simultaneous hyperpolarization of different substrates allows e.g. the study of molecule-molecule interactions and their binding behavior.
Introduction
MR signal enhancements can be achieved by using e. g. the hyperpolarization technique photo-Chemical Induced Dynamic Nuclear Polarization (photo-CIDNP).1,2 Previous studies have shown that 19F MR hyperpolarization in aqueous solutions is a challenge.3,4 Metabolism examination of fluorinated drugs as well as their intermolecular interactions are of high interest.5,6 To date, only photo-chemically induced dynamic nuclear polarization (photo-CIDNP) allows the 19F hyperpolarization in pure water.3,7 Here, we used a low-cost LED-based set-up.7-9Theory
In photo-CIDNP experiments, the polarization forms out of the reactions between radical pairs. The signal formation is determined by the radical and radical pair parameters such as hyperfine couplings and g-factors in the detection field. photo-CIDNP is strongly related to the singlet and triplet states, which are formed by the interaction of two electrons with spin-1/2. These radical pairs occur because of their limited motion in the solvent cage. Formation of radicals can be achieved e. g. by photolytic excitation of a precursor molecule (here: riboflavin 5’-monophosphate sodium salt hydrate). During the lifetime of the solvent cage the correlated radical pair can recombine (recombination/geminate products) or diffuse apart and pass to side reactions (escape/transfer products). The achieved nuclear hyperpolarization can also be transferred to heteronuclei e.g. 13C, 15N or 19F, among coupled spins.3Methods
In the current study, different fluorinated aromatic substrates were dissolved in aqueous solution. For example: The amino acids 2-fluoro-DL-tyrosine (2.4 mg) and 3-fluoro-DL-tyrosine (2.1 mg), as well as 2.2 mg of 3-fluoro-4-hydroxyphenylacetic acid (a compound the can be found in olive oil in the non-fluorinated form) were hyperpolarized in D2O, respectively physiologic salt solution. As photosensitizer riboflavin 5’-monophosphate sodium salt hydrate (1.2 mg) were dissolved in 6 ml physiologic salt solution. 600 µl of this stock solution were used in MR measurements (5 mm NMR tube). An optical fiber connected to a Cree XP E high power LED (455 nm, 3.4 V, ~400 mA) was centrally positioned in the solution. The irradiation was controlled via a self-written pulse program of a 7 T NMR spectrometer (Bruker WB-300 Ultrashield). Irradiation times were between 0 s and 15 s. 19F NMR spectra were measured using a 90° pulse (P1 = 32.5 µs, PL1 = 17 W).Results
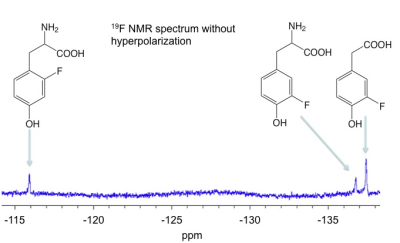
Figure 1 shows the 19F NMR spectrum in thermal equilibrium of a mixture of 2-fluoro-DL-tyrosine, 3-fluoro-DL-tyrosine and 3-fluoro-4-hydroxyphenylacetic acid. Without light irradiation only small signals can be detected.When switch on the LED, the signals of 3-fluoro-DL-tyrosine and 3-fluoro-4-hydroxyphenylacetic acid will be decreased. The signal amplification depends, among other things, on the concentration of the respective substrate and the photosensitizer used. It was found that with lower concentrations the signal amplification increases.7
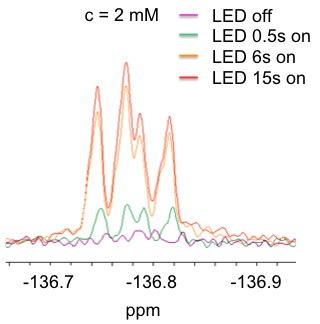
Figure 2 shows, for example, the signal enhancements in dependence of the irradiation time in the case of a 2 mM solution of 3-fluoro-DL-tyrosine in aqueous solution.
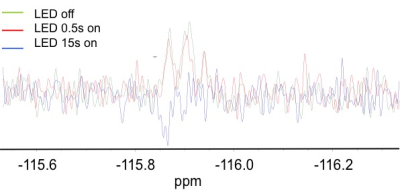
In the case of 2-fluorotyrosine no signal enhancement can be detected at a field strength of 7 T. To observe here is a sign change with increasing irradiation time. Thus, a polarization transfer to 19F is measured.
Further substrates that have been studied in our lab are 3-fluoro-4-hydroxybenzoic acid and 4-hydroxy-3-(trifluoromethyl)benzoic acid. 3-Fluoro-4-hydroxybenzoic acid shows comparable signal enhancements as 3-fluoro-DL-tyrosine and 3-fluoro-4-hydroxyphenylacetic acid.
Discussion
The results show that the hyperpolarization of 19F nuclei in aqueous solution is possible. With photo-CIDNP this is repeatable without adding a substrate again. The type or phase of the hyperpolarized signals is dependent on the substitution of the aromatic system and the magnetic field in which is measured. In addition, photo-CIDNP allows the simultaneous hyperpolarization of various substrates directly in the detection field. No further transfer or transport is necessary.Conclusion
Photo-CIDNP was shown to significantly enhance 19F NMR signals of different fluorinated aromatic systems. High signal enhancements were achieved for molecules such as 3-fluoro-DL-tyrosine or 3-fluoro-4-hydroxyphenylacetic acid. Low or no increase of 19F signals is observable for ortho-substituted aromatic rings (e. g. 2-fluoro-DL-tyrosine). The simultaneous hyperpolarization of different substrates directly inside the detection field allows a fast examination of molecule interactions as well as investigations of metabolisms.Acknowledgements
No acknowledgement found.References
1. Bargon J, et al. Kernresonanz-Emissionslinien während rascher Radikalreaktionen. Zeitschrift Naturforschung Teil A, 1967;22:1551-1555.
2. Goez M, Photo-CIDNP spectroscopy. Annual reports on NMR Spectroscopy, 2009;66:77-147.
3. Flögel U, Ahrens E, 1. Ed. , Fluorine Magnetic Resonance Imaging, Pan Stanford Publishing, Singapore, 2017.
4. Hövener JB, Pravdivtsev AN, Kidd B, et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed., 2018;57:11140 – 11162.
5. Khan F, Kuprov I, Craggs TD, et al. 19F NMR Studies of the Native and Denatured States of Green Fluorescent Protein JACS, 2006;128:10729–10737.
6. Ojima I, Fluorine in Medical Chemistry and Chemical Biology, 1.Ed., Wiley-Blackwell: Chichester, 2009.
7. Bernarding J, Euchner F, Bruns C, et al. Low-cost LED-based Photo-CIDNP Enables Biocompatible Hyperpolarization of 19F for NMR and MRI at 7 T and 4.7 T. ChemPhysChem., 2018;19:2453-2456.
8. Feldmeier C, Bartling H, Riedle E, Gschwind R.M. LED based NMR illumination device for mechanistic studies on photochemical reactions – Versatile and simple, yet surprisingly powerful. J. Magn. Res., 2013;232:39-44.
9. Feldmeier C, Bartling H, Magerl K, Gschwind R.M. LED-Illuminated NMR Studies of Flavin-Catalyzed Photooxidations Reveal Solvent Control of the Electron-Transfer Mechanism. Angew. Chem., 2015;127:1363-1367.
Figures