3032
Novel Real-time Hyperpolarized 13C Metabolic Tracer Probes γ-Glutamyl Transferase Activities in vivo Tumor Xenografts1National Institutes of Health, Bethesda, MD, United States, 2National Cancer Institute, National Institutes of Health, Bethesda, MD, United States, 3University of Tokyo, Bunkyo ku, Japan, 4Quantum Medical Science, Chiba, Japan, 5Technical University of Denmark, Lyngby, Denmark, 6Department of Electrical Engineering, Technical University of Denmark, Lyngby, Denmark
Synopsis
It is well known that dysregulation of γ-glutamyl transferase (GGT) activities in malignant cells leads to more aggressive phenotypes by producing reactive oxygen species. GGT is important for glutathione homeostasis, and has also been used as a diagnostic marker for various pathologies in the liver, biliary system, and pancreas. Here, for the first time, a novel hyperpolarized 13C probe, γ-Glu-[1-13C]Gly, was demonstrated in in vivo tumor xenografts, including human pancreatic ductal adenocarcinoma and ovarian adenocarcinoma, to detect real-time γ-glutamyl transferase activities as a prospective biomarker for monitoring the tumor progression and prognosis with/without various cancer therapeutic approaches.
Purpose
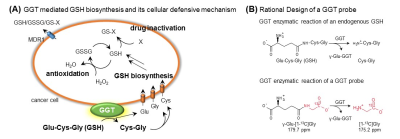
γ-glutamyl transpeptidase (GGT) is a membrane-associated enzyme and plays a crucial role in glutathione homeostasis and is used as a diagnostic marker for various pathologies in the liver, biliary system, and pancreas as shown Figure 1(A).1,2 Latent elevations in GGT can suggest chronic viral hepatitis, cirrhosis, cardiovascular disease, diabetes, pancreatitis, and cancer.1-3 In particular, it has been known that γ-glutamyl transpeptidase is associated with the progression of tumor and suggested as a promising candidate for a tumor biomarker.3 Overexpression of γ-glutamyl transpeptidase leads to intracellular glutathione production, which limits oxidative stress and facilitates the glutathione-dependent drug resistance.3,4 γ-glutamyl transpeptidase is frequently overexpressed in various tumor types, including soft tissue sarcoma and ovarian adenocarcinoma.4 Therefore, direct observation of γ-glutamyl transpeptidase activities provides tremendous potential for diagnosis and monitoring treatment responses. Despite the importance of this tumor biomarker, the direct imaging of these activities using traditional fluorescence or radioactivity measurements has been hampered by their limitations, and there are no imaging modalities that can provide in vivo real-time information.5 Here, in order to monitor these informative enzymatic activities in real-time, hyperpolarized γ-Glu-[1-13C]Gly was applied using in vivo 13C MRI (Figure 1(B)). The family of this promising probe were demonstrated on tumor bearing xenografts, including human pancreatic ductal adenocarcinoma and ovarian adenocarcinoma, with/without various cancer therapeutic approaches.Methods
Tumor xenograft model:2×106 cells of human pancreatic ductal cancer cell line, MiaPaCa-2 was subcutaneously inoculated into the hind legs of female athymic nude mice.
In vivo HP 13C experiments:
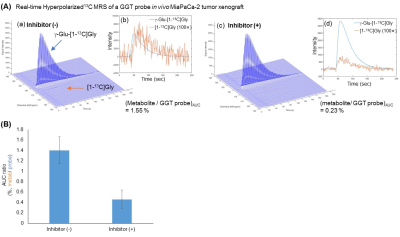
GGT probe, γ-Glu-[1-13C]Gly was dissolved in 5 M sodium hydroxide (NaOH) aqueous solution resulting 2.6M γ-Glu-[1-13C]Gly solution. γ-Glu-[1-13C]Gly 80 uL, containing OX063 17.5 mM and Gd 0.94 mM was hyperpolarized at 3.35 Tesla and 1.4 K using the Hypersense. After 90 to 120 minutes of polarizations, the hyperpolarized sample was dissolved in 4.0 mL of a superheated acidic buffer that consists of 50 mM Tris(hydroxymethyl) aminomethane, 10 mM HCl, and 100 mg/L ethylenediaminetetraacetic acid. The 52 mM of hyperpolarized γ-Glu-[1-13C]Gly solution was intravenously injected through a catheter placed in the tail vein of the mouse (300 μL/mouse). Hyperpolarized 13C single pulse MR spectra were recorded with a 3-Tesla scanner (MR Solutions, Guildford, UK), a spectral width of 2.5 kHz, FID points of 256, repetition time of 1000 ms, and flip angle of 10 degrees over 240 seconds. The signal processing and displays were conducted using MATLAB. In order to inhibit GGT, GGsTop (5 mg/ kg) was intravenously administered 1 hour before HP-MRS measurement. Area under the curve ratios of metabolite and parent probe were compared between mice with/without a GGT inhibitor group (n=3) and estimated the significance using unpaired t-test (equal variance).
Results and Discussions
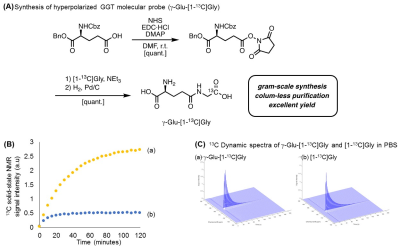
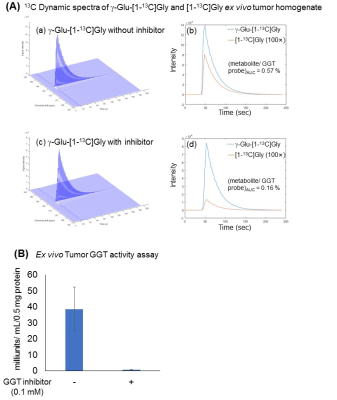
A superior synthesis scheme, which utilized an optimized condensation reagent and recrystallization, allowed us to produce the industry scale of γ-Glu-[1-13C]Gly, as shown in Figure 2(A). This larger scale production of 13C labeled material is greatly advantageous for in vivo hyperpolarized 13C MRI. These highly purified probes were applied to the solid-state polarization, which was drastically enhanced due to our improved sample preparation approach without using conventional glycerol mixtures as a glassing agent as shown in Figure 2(B). Using this optimized conditions, the dynamic MR spectra of both γ-Glu-[1-13C]Gly and its enzymatic product [1-13C]Gly were acquired in Figure 2(C). γ-glutamyl transpeptidase cleaves the γ-peptide bond between the glutamyl and cysteinyl groups of glutathiones, therefore, the product of this enzymatic activities of γ-Glu-[1-13C]Gly would be smaller size of [1- 13C]Gly, which has longer T1 relaxation time. In order to demonstrate further feasibilities, the GGT probe was applied to human pancreatic, MiaPaCa-2 tumor xenografts, which have relatively higher expression of GGT among pancreatic cancer cell lines.6 Hyperpolarized γ-Glu-[1-13C]Gly consistently produced its enzymatic product [1-13C]Gly upon GGT activities on a MiaPaCa-2 tumor extract as well as in vivo tumor xenografts as shown in Figure 3 and Figure 4. The enzymatic conversion of [1-13C]Gly was notably suppressed upon the administration of a GGT inhibitor, which indicates that the γ-Glu-[1-13C]Gly can be a functional readout of GGT mediated metabolism in in vivo tumors as shown in Figure 3(B) and 4(B).Conclusions
In this study, robust real-time monitoring of in vivo GGT activities in tumor xenografts, and their responses towards therapeutics were successfully demonstrated. Comparing to other existing in vivo hyperpolarization probes, this novel probe has following advantages: (1) both the probe γ-Glu-[1-13C]Gly and the product [1-13C]Gly have relatively long lifetime as hyperpolarized 13C probes (~30 seconds and ~45 seconds, respectively), (2) the 13C chemical shift difference between the probe and the product is sufficiently large enough (~ 4.3ppm) to distinguish between these two resonances at physiological pH, (3) this probe has lower toxicity when infused at millimolar concentrations, and (4) the enzymatic domain of membrane-associated g -glutamyl transpeptidase is located on the extracellular surface of cells, therefore, the hyperpolarized substrates can access to the reaction center of the GGT readily.Acknowledgements
This study was supported by intramural research program at NCI/NIH.References
[1] Keillor JW et al., Gamma-glutamyl transpeptidase substrate specificity and catalytic mechanism. Methods Enzymol. 2005;401:449–467.
[2] Zhang H et al., Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–483.
[3] Fentiman IS et al., Gamma-glutamyl transferase: risk and prognosis of cancer. Br. J. Cancer. 2012;106:1467-1468.
[4] Hanigan MH, Gamma-Glutamyl Transpeptidase: Redox Regulation and Drug Resistance Adv. Cancer Res. 2014;122:103–141.
[5] Urano Y et al., Rapid Cancer Detection by Topically Spraying a γ-Glutamyltranspeptidase–Activated Fluorescent Probe. Sci. Trans. Med. 2011;3:110-119.
[6] Ramsay EE et al., Employing Pancreatic Tumor γ‑Glutamyltransferase for Therapeutic Delivery. Mol. Pharm. 2014;11:1500−1511.
Figures