3031
A fast, single-shot narrowband excitation method for resolving J-modulated artifacts in hyperpolarized [2-13C] Lactate imaging1Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Magnetic resonance spectroscopic imaging of hyperpolarized [2-13C] Pyruvate and its metabolic products enables real-time assessment of in vivo glycolysis and oxidative phosphorylation simultaneously. However, significant technical difficulties arising from the widely dispersed spectrum and the J-coupling of the 13C labeled carbon with its chemically bonded proton limit its practical use for imaging. We propose a narrowband radiofrequency excitation based-method for eliminating the J-modulated artifacts in imaging [2-13C] Lactate. Results from simulations, phantoms and rat brain demonstrate the utility of this method, and highlight its potential role in the real-time simultaneous assessment of GLY and OXPHOS in vivo.
Introduction
Magnetic resonance spectroscopy of hyperpolarized [2-13C] pyruvate (pyr) and its metabolic products is a powerful tool to study real-time in vivo glycolysis (GLY) as [2-13C] pyruvate is converted to [2-13C] lactate (lac), as well as oxidative phosphorylation (OXPHOS), as the 13C label is retained in tricarboxylic acid (TCA) cycle compounds citrate, glutamate and acetylcarnitine, with applications in studying cellular metabolism, tumor metabolism, response to therapy and cardiac energetics1-3. Despite this advantage of using [2-13C] pyr to obtain a direct measure of OXPHOS and GLY simultaneously, the large chemical shift dispersion of [2-13C] pyr and its metabolites, the resulting chemical shift displacement artifact during slice excitation, the dipolar relaxation & J-coupling effects caused by the proton attached to the C2 carbon limit its practical use. Previously we presented a hybrid technique that utilizes fully resolved spectroscopic imaging to assess mitochondrial metabolites and a two-shot quadrature imaging method to image [2-13C] lac4. In this work, we propose a faster single-shot narrrowband radiofrequency (RF) excitation method for resolution of J-modulated artifacts arising from the [2-13C] lac doublet. Using product operator formalism (POF) we show that the combination of an in-phase and an anti-phase doublet due to the long duration of the narrowband excitation pulse resolves the J-modulated ghosting artifact. Results from simulations, phantoms and rat brain are presented that highlight the utility of this method for potential real-time simultaneous assessment of GLY and OXPHOS in vivo.Theory
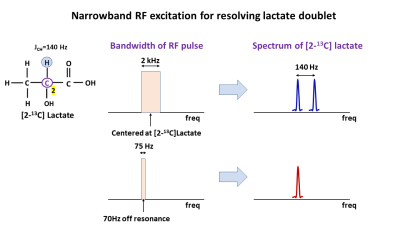
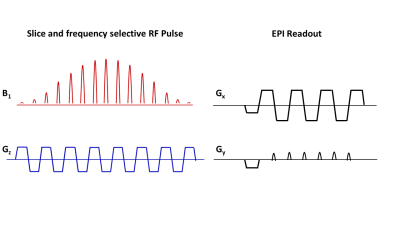
The starting state of a weakly coupled I-S spin system $$$\hat{\rho} = \hat{I}_{z}+\hat{S}_{z}$$$ with C2 carbon as the I-spin and the attached proton as the S-spin, in the presence of a narrow bandwidth off-resonance radiofrequency field evolves under the Hamiltonian (rotating frame of reference): $$\hat{H}=-{\Omega_{I}}\hat{I}_{z}-{\Omega_{S}}\hat{S}_{z}-{\omega_{1}}\hat{I}_{x}+2{\pi}J\widehat{I_{z}{S}_{z}} \qquad \qquad \qquad[1]$$ where $$$\Omega_{I}$$$, $$$\Omega_{S}$$$ are the off-resonance frequencies (rad/s) of the I & S spins respectively, J (Hz) is the J-coupling constant between the I and S spins and $$$\omega_{1}$$$ is the frequency (rad/s) of the applied radio frequency pulse. After a 900 RF pulse, the spin density operator evolves to: $$\hat{\rho}=\frac{1}{2}\hat{I}_{y}+\frac{1}{2}\hat{I}_{z}+\frac{1}{2}2\widehat{I_{y}{S}_{z}}-\frac{1}{2}2\widehat{I_{z}{S}_{z}}+\hat{S}_{z}\qquad \qquad \qquad [2]$$ The sum of the in-phase doublet arising from the $$$\hat{I}_{y}$$$ term combines with anti-phase doublet from $$$2\widehat{I_{y}{S}_{z}}$$$ to produce a singlet (Fig 1), eliminating all J-coupling associated artifacts during signal acquisition. Therefore, a narrowband spectrally and spatially selective pulse with a spectral bandwidth (BW) of 75Hz centered 70Hz (J/2) away from the [2-13C] lac resonance, followed by a fast echoplanar imaging (EPI) readout, can be used to image [2-13C] lac artifact free (Fig 2).Methods
Simulation & in vitro experiments:A full-density matrix simulator for a coupled 2-spin I-S system in MATLAB (Mathworks Inc., MA, USA) developed in-house was used to simulate the evolution of coherences. A syringe filled with 5ml of 2.5M 13C formate(dia. 15mm), doped with 8μl/ml of Gadolinium agent to shorten the in-vitro T1, was placed in a 13C transmit-receive surface coil (dia ~35mm) and imaged using a clinical 3T PET-MR scanner(GE Medical systems). A narrowband spectrally selective radiofrequency excitation pulse(BW=100Hz,TRF=40ms,slice thickness=20mm,100Hz off-resonance), followed by free-induction decay signal acquisition(BW=5000Hz,N=2048,TR=2s), was used to test the narrowband technique for eliminating the splitting from J-coupling. Another syringe filled with 0.5M [2-13C] lac was used in the EPI imaging experiment.Hyperpolarization and in vivo imaging of [2-13C] lac: 54mL of 15.5M [2-13C]Pyruvic acid (Sigma Aldrich) mixed with 15mM trityl radical AH11141(GE Medical systems) was polarized between 3-4 hours in SPINLab system(GE Medical systems) and dissolved using 16g solution of 40mM tris(hydroxymethyl aminomethane),100mg/L ethylenediaminetetraacetic acid(EDTA), and 50mM NaCl. The resulting 100mM pyruvate solution was neutralized with 640mL of 125mM NaOH buffer. 2.5ml of this solution was injected at a rate of 0.25mL/s into a male Wistar rat through a tail-vein catheter and imaged in a clinical 3T scanner(Signa MR750,GE Systems) 20s post injection to maximize the signal from lactate. A slice and frequency selective narrowband RF pulse centered on one of the lac peaks(slice-thickness=10mm, BW=125Hz,pulse-width=25.6ms,flip-angle=900) followed by an EPI readout (FOV=128mm,matrix=32x32, TR=200ms,TE=24ms) was used to acquire the [2-13C] lac image from an axial slice placed in the rat-brain, and this was overlaid on a T2-weighted proton image acquired using a custom-built 1H T/R bird-cage coil for anatomical reference (FOV=128mm,256x256 matrix,2mm slice-thickness,TR/TE=6000/125ms).
Results
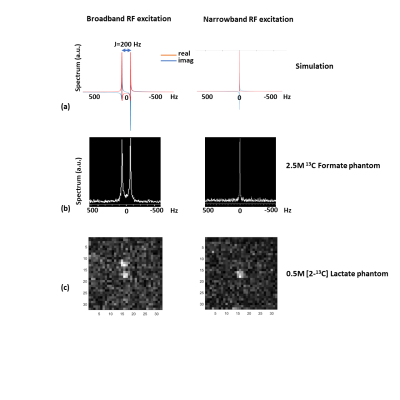
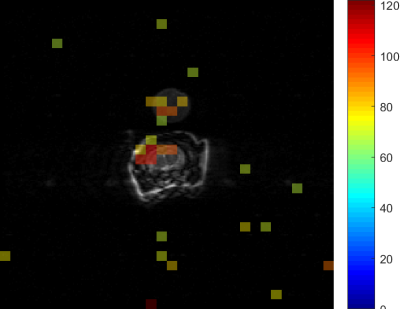
Spectrum from the numerical simulation as well as the in-vitro 13C formate using full bandwidth RF pulse(2kHz, 1.8ms) demonstrates the splitting caused due to the J-coupling between the 13C labeled carbon and its attached proton (left panel, Fig 3a & 3b respectively). Use of a narrow bandwidth RF pulse (100Hz, 40ms) results in a singlet as seen in the simulation (Fig 3a, right panel) and the in-vitro spectrum (Fig 3b, right panel). This singlet can then be imaged using a single-shot fast imaging EPI readout as seen in Fig 3c ([2-13C] lac phantom). An artifact-free in vivo [2-13C] lac map acquired from a rat brain after an injection of hyperpolarized [2-13C] pyr, and overlaid on a T2-weighted image is shown in Fig 4.Conclusion
We propose a fast single-shot narrowband RF excitation based imaging method to resolve the artifacts due to the J-coupling evolution during signal collection of [2-13C] lac and show that it can facilitate simultaneous in vivo measurement of glycolysis and mitochondrial metabolism during a hyperpolarized [2-13C] pyr experiment.Acknowledgements
The Lucas Foundation. National Institutes of Health (NIH) grants R01 EB019018, R01 CA176836, P41 EB015891.
References
1. Schroeder MA, Atherton HJ, Ball DR, et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB J 2009;23(8):2529–2538.
2. Park JM, Josan S, Grafendorfer T, et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2-¹³C] pyruvate. NMR Biomed 2013;26(10):1197–1203.
3. Dodd MS, Atherton HJ, Carr CA, et al. Impaired in vivo mitochondrial Krebs cycle activity after myocardial infarction assessed using hyperpolarized magnetic resonance spectroscopy. Circ Cardiovasc Imaging 2014;7(6):895–904.
4. Datta K, Spielman DM. A Quadrature Detection Technique to Resolve J-Modulated [2-13C]Lactate in Hyperpolarized [2-13C] Pyruvate Imaging. program # 4299. ISMRM 27th annual meeting and exhibition, 2019, Montreal, Canada.
Figures