3028
ROKET: a Robust Keto Enol Tautomerisation phantom for multi-site, multi-vendor hyperpolarized 13C studies1Oxford Centre for Clinical Magnetic Resonance Research (OCMR), University of Oxford, Oxford, United Kingdom, 2Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 3Department of Anatomy and Physiology, University of Oxford, Oxford, United Kingdom, 4Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 5Department of Clinical Medicine, the MR Research Centre, Aarhus University, Aarhus N, Denmark, 6CRUK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 7Department of Radiology, University of Cambridge, Cambridge, United Kingdom
Synopsis
As dissolution dynamic nuclear polarization (d-DNP) hyperpolarised 13C magnetic resonance imaging progresses towards multi-centre clinical trials, a reproducible, scalable in vitro testing platform is required that allows quality assurance, multi-centre validation, and further technical development. We present a method to exploit the inherent pH dependence of the hydrate-to-keto ratio of pyruvic acid to generate a simple, inexpensive, and reproducible non-enzymatic dynamic phantom to address this need. The method was validated at three different sites with a variety of hardware produced by different vendors.
Introduction
Hyperpolarized 13C magnetic resonance is an emerging investigational technique that enables the non-invasive measurement of metabolic rates in vivo through the preparation of a pre-polarized injectable substrate.1 As the clinical translation of this technology progresses towards multi-centre trials, a reproducible, scalable and inexpensive in vitro testing platform is required to enable accurate calibration and comparison of dynamic measurements.2 Preparation, administration, and measurement of the metabolic conversion of a polarized substrate are complex, multi-facetted processes which are difficult to replicate in a conventional phantom. Robust enzyme-based phantoms that allow rate constants to be validated are challenging and require precise temperature control, accurate storage information, and specialised hardware in order to be reproducible.3 Here we present a simple dynamic phantom that uses standard clinical hardware and the intrinsic chemical tautomerization of pyruvic acid to reproducibly emulate label exchange kinetics. The phantom was validated at three different sites using scanners from two different vendors.Theory
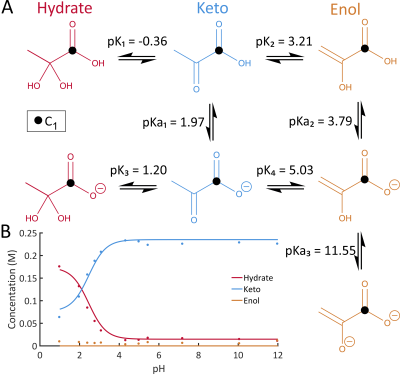
Pyruvic acid exhibits rapid tautomerization between keto, enol, and hydrated forms4 as well as equilibrium between protonated and deprotonated forms. The dominant form varies with pH (Figure 1). In acidic conditions, the keto and hydrate forms exist at approximately equal concentrations. Increasing pH makes the hydrated form less favourable with a ratio of ~1:12 hydrated:keto observed at physiological pH (Figure 1B).5 Therefore, the controlled infusion of acid into a neutralized solution of pyruvic acid will alter the hydrate:keto ratio on the timescale of a hyperpolarized experiment, emulating kinetic changes observed in vivo in addition to T1 decay. These kinetic changes can be fit and parameterized similarly to those seen during enzymatic reactions, but are inherently a function of pH which is easily controlled experimentally by the acid infusion rate.Methods
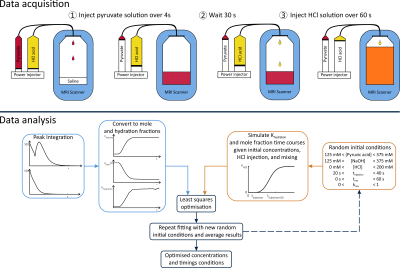
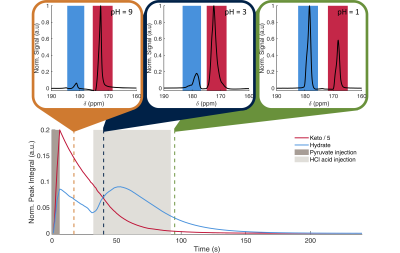
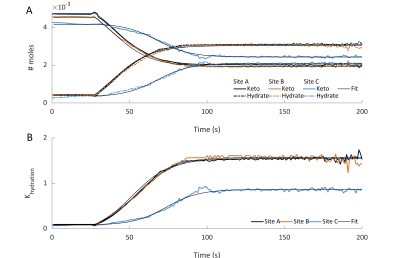
A mixture of [1-13C]pyruvic acid (Sigma-Aldrich) and 15 mM EPA (AH111501, Syncom) was hyperpolarized in a 5T SPINlab polariser (GE Healthcare) and dissolved into sodium hydroxide (Sigma-Aldrich) to yield a neutralized 250 mM solution. 20 mL of the neutralized solution was injected over 4 s into 50 mL of 0.9% NaCl in a 250 mL bottle (BBraun Medical Ltd) using a power injector (MedRad Spectris Solaris, Bayer). 110 mL of 0.1 M HCl solution (Sigma-Aldrich) was loaded into the flush syringe of the power injector. 7 mL of HCl was used to flush the tubing immediately after the pyruvate injection to clear the line without adding HCl to the bottle. 30 s later, 100 mL of 0.1 M HCl was infused over one minute from the flush syringe of the power injector. Non-localized spectra (TR = 1 s, FA = 10°, bandwidth = 5 kHz, 2048 complex points, 240 measurements) were acquired. This process was repeated 3 times at 3 different sites equipped with: (A) 3T Trio (Siemens) with a quadrature birdcage 1H/13C head coil (Rapid Biomedical), (B) 3T MR750 (GE Healthcare) with the same design of head coil (Rapid Biomedical), and (C) 3T Signa HDx (GE Healthcare) with a clamshell volume 13C transmitter (GE Healthcare) and 10 cm loop receiver (Rapid Biomedical). All data were processed in MATLAB according to the schematic shown in Figure 2. Briefly, keto and hydrate signals were integrated and normalised to total carbon signal to give the mole fraction. This was converted to the number of moles using the concentration of pyruvic acid measured after dissolution. This data was fitted to a forward model of the chemical system describing the concentrations of the different species, timings of injections, and mixing using a least square method (Figures 1 and 2). The full processing pipeline is available at: github.com/roket.Results and discussion
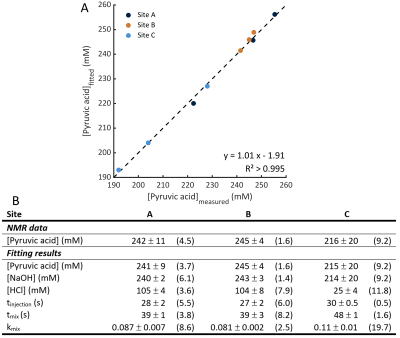
Examples of the kinetic curves generated and the associated fits are shown in Figures 3 and 4 respectively. A summary of all fitting results are shown in Figure 5. The model manages to accurately calculate the initial pyruvic acid concentration with an R2 > 0.995 between the fitted concentration and the concentration measured by high-resolution NMR (Figure 5A). Intra-site reproducibility was very high with coefficients of variation less than 10% in all fitted parameters for Sites A and B (Figure 5B). Site C showed higher variation in the fitted parameters, although the level of variation was similar to the levels of variation in the pyruvic acid concentration injected. Inter-site reproducibility was very high between Sites A and B (<5 % difference in all parameters) but lower between A and C (<75% difference) or B and C (<75% difference). This was likely a result of the large differences in pyruvic acid concentration seen at site C as well as an apparent 4-fold difference in the strength of acid used at this site.Conclusion
As the clinical translation of hyperpolarisation technology matures, there is an emerging need for a reproducible in vitro platform capable of making QA assessments and comparative validation in multi-centre clinical trials. This platform is also needed to facilitate technical development of new hardware and pulse sequences without the dependence on pre-clinical models for early studies. We have shown the feasibility of exploiting intrinsic acid/base chemistry to fulfil this need and produce kinetic curves that can be reproduced at multiple sites across a range of different hardware.Acknowledgements
This work was funded by NIHR Oxford Biomedical Research Centre and the British Heart Foundation. CTR is funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society [098436/Z/12/B].References
1. Ardenkjaer-Larsen, J.H., et al., Dynamic nuclear polarization polarizer for sterile use intent. NMR in Biomedicine, 2011. 24(8): p. 927-932.
2. Kurhanewicz, J., et al., Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia, 2019. 21(1): p. 1-16.
3. Walker, C.M., et al., A Catalyzing Phantom for Reproducible Dynamic Conversion of Hyperpolarized [1-13C]-Pyruvate. PLOS ONE, 2013. 8(8): p. e71274.
4. Chiang, Y., A.J. Kresge, and P. Pruszynski, Keto-enol equilibria in the pyruvic acid system: determination of the keto-enol equilibrium constants of pyruvic acid and pyruvate anion and the acidity constant of pyruvate enol in aqueous solution. Journal of the American Chemical Society, 1992. 114(8): p. 3103-3107.
5. Golman, K., R. in 't Zandt, and M. Thaning, Real-time metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America, 2006. 103(30): p. 11270-11275.
Figures