3024
Feasibility of Hyperpolarized [89Y]-Labeled Substrates for In Vivo Studies1AIRC, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2Physics, University of Texas at Dallas, Richardson, TX, United States, 3Electrical Engineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Yttrium-89 in the form of diamagnetic 89Y complexes is an attractive nucleus for the design of responsive magnetic resonance spectroscopy and imaging probes the 89Y NMR chemical shift is quite sensitive to the coordination environment of the Y3+ ion. 89Y has a great potential for in vivo hyperpolarized NMR due to its extremely long T1 relaxation time. In this study, we developed an RF coil for imaging 89Y signal in vivo, tested it with phantoms containing thermally polarized and hyperpolarized yttrium compounds, and demonstrated the feasibility of using hyperpolarized 89Y complexes for in vivo studies in a rat.
Introduction
Nuclear magnetic resonance (NMR) has an inherently low sensitivity. Hyperpolarization generally refers to methods that aim at improving the sensitivity of NMR by creating non-equilibrium nuclear spin distribution on the Zeeman levels. The hyperpolarized (HP) magnetization will decay according to T1 and the time constraint emerging from the inevitable decay of polarization is a severe limitation of HP-NMR technology. 89Y (½ spin g = -2.0864 MHz/T, 100 % natural abundance) has one of the longest T1 values among the NMR active nuclei. The long T1 of 89Y (up to 600 s) translates into a much longer polarization lifetime than that of hyperpolarized 13C allowing the observation of chemical or biological processes on a longer timescale. 89Y is a very attractive nucleus for the design of responsive magnetic resonance spectroscopy and imaging probes because of the sensitivity of the 89Y NMR chemical shift to changes in the coordination environment of the Y3+ ion. Most importantly, Y3+ is a pseudolanthanide (its ionic radius is about the same as that of Ho3+) and so ligands developed for conventional Gd(III)-based (T1-shortening) or Eu(III)-based (PARACEST) MRI agents can directly be applied with Y(III). Earlier we have shown that hyperpolarized 89Y-DOTP, (DOTP = 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetramethylenephosphonic acid) can be used as a 89Y MR spectroscopy probe to measure pH (1). The low gyromagnetic ratio makes 89Y one of the most challenging nuclei for conventional NMR and MRI. However, we have shown that 89Y-DOTA (DOTA = 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid, Fig.1) can be hyperpolarized by dissolution dynamic nuclear polarization (d-DNP) using commercially available hardware. We have achieved over 60,000-fold 89Y NMR signal enhancement of 89Y-DOTA after optimization of the DNP conditions. The 89Y T1 was found to be 499 s at 9.4 T. (2, 3). Despite its great potential, HP 89Y has not been tested in vivo. The goal of this project was to study the feasibility of detecting an 89Y NMR signal in vivo after the injection of HP 89Y-DOTA into rats.Methods
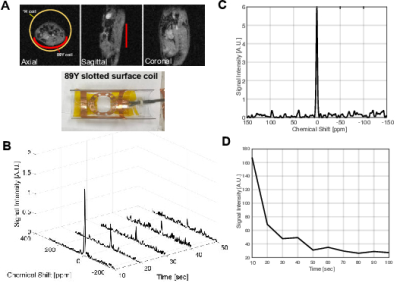
Both in vitro phantom study and in vivo MR spectroscopy were performed at Agilent 4.7T animal MR scanner. We updated the ‘macroprescan_tn’ file to match the nuclear and atomic number of 89Y nucleus. A custom-built 89Y slotted surface coil (4) (Ø = 60 mm, 9.806 MHz) was placed under the phantom or the abdominal area of the rats for both radiofrequency (RF) excitation and data acquisition. In order to obtain the 9.806 MHz signals, the preamplifier was linked with a quarter wave cable. First, a glass-vial phantom containing gadolinium-doped (ProHance, 5 mM) 89YCl3 (2 M) was used for testing and calibrating the system. For in vivo studies, 89Y-DOTA was synthesized as sodium salt (Fig.1). A HyperSense polarizer (3.35T, Oxford Instruments Molecular Biotools, UK) was used for 89Y-DNP. 89Y-DOTA (0.5 M in 3:1 w/w water:glycerol matrix doped with trityl OX063 polarizing agent at 15-mM) was polarized for 3 hours followed by dissolution with 4 mL of superheated water to give a solution of HP-89Y-DOTA (11 mM final concentration). The HP-89Y-DOTA solution was administered to healthy male Fischer rats (~200 g) by intravenous injection through the tail vein as a bolus (0.125 mmol/kg body weight, up to 4.0 mL, injection rate = 0.25 mL/s), immediately followed by a dynamic 89Y MRS scan (pulse-and-acquire with 90o hard pulse RF excitation and repetition time = 10 sec).Results and Discussion
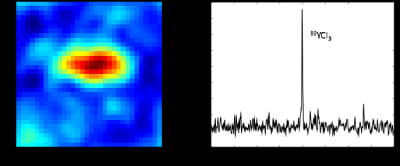
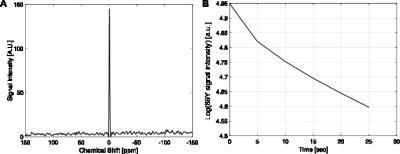
We designed a slotted surface radiofrequency (RF) coil for imaging 89Y signal in a 4.7 T animal MR system. The coil has improved penetration and improved signal to noise ratio over the area of interest compared to regular surface coils (4). Due to the relatively long distance between the polarizer and the scanner and the effects of spatially fluctuating fringe magnetic fields from several high-field systems along the delivery path, relatively weak HP 89Y signals were detected in both in vitro and in vivo experiments at the 4.7 T scanner. The chemical shift of 89YCl3 was set as reference (0 ppm). The capability of detecting 89Y signals at the scanner was demonstrated by the chemical shift imaging (CSI) of thermally-polarized 89YCl3 phantom (FOV = 65 x 65 mm2, matrix = 8 x 8, nominal flip-angle = 20o, 10 averages), Fig 2. After confirming that HP 89Y-DOTA could be detected at 4.7 T with the slotted surface coil with 10° pulse (Fig.3), we managed to detect in vivo signal of hyperpolarized 89Y-DOTA at 109 ppm from rat abdominal area with 90° pulse (Fig.4).Conclusion
In summary, we developed a slotted surface coil for detecting 89Y resonance in a 4.7 T animal MR scanner, and showed 89Y signal could be detected in phantoms containing thermally polarized 89YCl3 and HP 89Y-DOTA solutions. We also demonstrated that in vivo HP 89Y signal could be observed after the injection of HP-89Y-DOTA into a rat. These results pave the way for the in vivo testing of responsive 89Y complexesAcknowledgements
UT Dallas Collaborative Biomedical Research Award; The Welch Foundation (I-2009-20190330); National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Texas Institute of Brain Injury and Repair.References
1. Jindal AK, Merritt ME, Suh EH, Malloy CR, Sherry AD, Kovacs Z. Hyperpolarized 89Y complexes as pH sensitive NMR probes. J Am Chem Soc. 2010;132(6):1784-5.
2. Lumata L, Jindal AK, Merritt ME, Malloy CR, Sherry AD, Kovacs Z. DNP by thermal mixing under optimized conditions yields >60,000-fold enhancement of 89Y NMR signal. J Am Chem Soc. 2011;133(22):8673-80.
3. Merritt ME, Harrison C, Kovacs Z, Kshirsagar P, Malloy CR, Sherry AD. Hyperpolarized (89)Y offers the potential of direct imaging of metal ions in biological systems by magnetic resonance. J Am Chem Soc. 2007;129(43):12942-3.
4. Solis SE, Wang R, Tomasi D, Rodriguez AO. A multi-slot surface coil for MRI of dual-rat imaging at 4 T. Phys Med Biol. 2011;56(12):3551-61.
Figures