3023
Radical-free and metal-free hyperpolarized MRI using endogenous pyruvate analogues1Laboratory for Functional and Metabolic Imaging (LIFMET), EPFL, Lausanne, Switzerland, 2Department of Diagnostic and Interventional Radiology, CHUV, Lausanne, Switzerland, 3Department of Diagnostic and Interventional Radiology, UNIL, Lausanne, Switzerland
Synopsis
Using nonpersistent radicals generated by UV-irradiation of endogenous metabolite precursors for dissolution DNP avoids the need for radical filtration and may potentially lengthen the measurement window of hyperpolarized MRI measurements. Here, the endogenous pyruvate-analogues alpha-ketobutyrate and alpha-ketovalerate were proposed as nonpersistent radical precursors. Radical yields were characterized along with their performance as polarizing agents for in vitro and in vivo dDNP experiments. A 13C-glucose liquid state polarization of 26.4% was attained using alpha-ketobutyrate-derived radical, while pyruvate-derived radical yielded 21.7% (compared to 18.9% reported with the persistent trityl radical). Alpha-ketobutyrate was used to hyperpolarize [1-13C]butyrate and measure cardiac metabolism in vivo.
Introduction
Hyperpolarization via dynamic nuclear polarization (DNP) enables a many-fold increase in the MR signal1 but the process requires free radicals polarizing agents. This poses two major challenges for clinical translation: 1) free radicals shorten the longitudinal relaxation time of 13C nuclei after dissolution2 and affect the already short duration of the hyperpolarized state; 2) free radicals require filtration prior to injection which is a time-consuming process, and further shortens the measurement window3. The use of nonpersistent radicals generated by UV irradiation such as pyruvic acid (PA)4,5,6, phenylglyoxylic acid7 and (d9)-trimethylpyruvic acid8 may address both challenges. The aim of this study was to investigate the endogenous pyruvate analogues alpha-ketobutyrate ($$$\alpha$$$kB) and alpha-ketovalerate ($$$\alpha$$$kV) as nonpersistent radical precursors for dissolution DNP and provide a comparison with PA.Methods
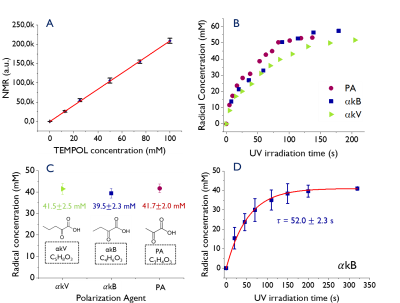
Sample preparation: (I) For radical characterization, PA, $$$\alpha$$$kB or $$$\alpha$$$kV were mixed in 1:1 glycerol:water (GW1:1). (II) For Solid State (SS) and Liquid State (LS) measurements, 2M [U-13C, U-2H]glucose was mixed with GW1:1 and 33%, 60% and 11% volume fractions of PA, $$$\alpha$$$kB or $$$\alpha$$$kV were admixed (n=5, Fig.1c). (III) For in vivo measurements, 0.66mmol [1-13C]-butyric acid (BA*) of volumetric composition $$$\alpha$$$kB:GW1:1:BA*=3:4:2 was mixed.All samples were sonicated at 50°C for 20min prior to freezing 7$$$\mu$$$l droplets in liquid nitrogen to create glassy beads and then irradiated with UV light for 200s with a DymaxBlueWave200 UV-lamp using a home-built setup6. Preparations were optimized to generate approximately 40mM final radical concentration.
Electron Spin Resonance (ESR): X-band ESR at 77K was used to estimate radical yield as a function of UV irradiation time. Absolute radical concentration was determined using a calibration curve with 0-100mM TEMPOL dissolved in GW1:1 (n=4, Fig.1a). Radical concentration build-up times were calculated using a mono-exponential fit (n=4, Fig.1d).
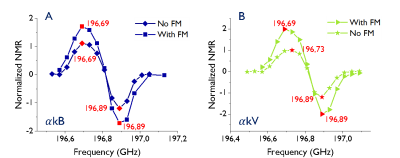
Hyperpolarization with DNP: Samples were hyperpolarized in a 7T home-built polarizer for 2hrs. Microwave (MW) frequency sweeps were conducted with and without MW frequency modulation (FM) to establish conditions for maximum DNP efficiency.
MRS: After dissolution of the hyperpolarized samples, LS and in vivo measurements were performed at 9.4T. Hyperpolarized 13C spectra were acquired 3s after dissolution using a 5° RF excitation pulse. Thermal equilibrium 13C spectrum was acquired using a 90° RF excitation pulse, Repetition Time (TR) of 60s with 64 averages. The enhancement $$$\epsilon$$$ was calculated as ratio of hyperpolarized and thermal signal intensity referring to carbon position C1 and polarization as $$$P=\epsilon*tanh(\hbar\gamma_C B_0/2k_B T)$$$. Hyperpolarized in vivo experiments were performed in male Wistar rats to measure cardiac metabolism as described in [5] and were approved by the local regulatory body.
Results
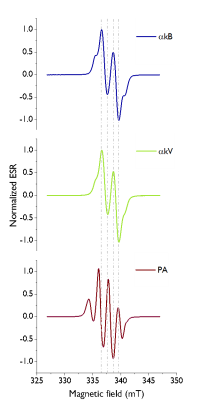
ESR spectra of the UV generated radicals in $$$\alpha$$$kB and $$$\alpha$$$kV are similar and narrower compared with PA (Fig.2).Following UV irradiation PA, $$$\alpha$$$kB and $$$\alpha$$$kV yielded radical concentrations of 55mM, 57mM and 54mM respectively. A plateau was observed after 200s (Fig 1b). The radical generation build-up time constant for UV-irradiated $$$\alpha$$$kB+[U-13C,U-2H]glucose was 52.0$$$\pm$$$2.3s (n=4, Fig.1d). MW frequency sweeps with and without FM showed that the polarization level of the $$$\alpha$$$kV-glucose sample was doubled by FM and the $$$\alpha$$$kB-glucose sample gained 50% (Fig.3).
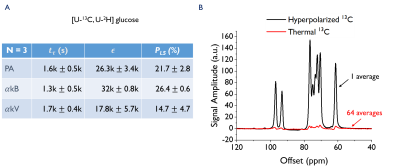
Liquid state polarization of 13C glucose was 21.7±2.8% for PA, 26.4±0.6% for $$$\alpha$$$kB and 14.7±4.7% for $$$\alpha$$$kV. Solid state build-up times were similar for all three samples (Fig.4, with spectra for $$$\alpha$$$kB).
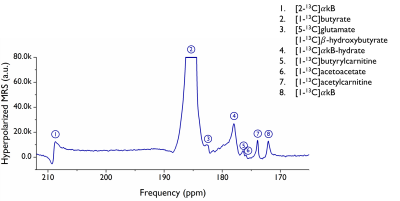
The [1-13C]butyrate-$$$\alpha$$$kB samples had a polarization build-up time of $$$t_\tau$$$=3.3k$$$\pm$$$0.3k s (n=5). Cardiac metabolism resulted in 13C labeling of [1-13C]acetylcarnitine, [1-13C]acetoacate and [1-13C]butyrylcarnitine. The natural abundance 13C resonances of C1 $$$\alpha$$$kB, C1 $$$\alpha$$$kB-hydrate and C2 $$$\alpha$$$kB were also observed (Fig.5).
Discussion
Two promising endogenous polarizing agents were studied for radical-free dissolution DNP. The use of $$$\alpha$$$kB increased the polarization of 13C glucose (26.4%) compared with PA (21.7%), and was 40% higher than previously reported using the persistent trityl radical Ox0639. Although UV-irradiated $$$\alpha$$$kB and $$$\alpha$$$kV demonstrated similar ESR lineshapes and radical yield in a neat GW1:1 matrix, adding glucose or butyric acid required a unique sample composition for each. Unexpectedly, $$$\alpha$$$kV, which is self-glassing, performed worse than PA, although the ESR linewidth was narrower. This illustrates that sample formulation requires a careful optimization in terms of UV-generated radical yield and polarization level for each 13C labelled metabolic substrate, which is still a largely empirical and nontrivial process. Compared to previous work using 13C-butyrate5, a different UV source, polarizing agent and the use of microwave modulation contributed to improved polarization levels leading to the detection of an increased number of metabolites.Conclusion
The pyruvate analogues $$$\alpha$$$kB and $$$\alpha$$$kV were proposed as endogenous polarizing agents for dissolution DNP. $$$\alpha$$$kB generated 26.4% liquid state polarization on 13C glucose and was successfully used in vivo to measure cardiac metabolism of [1-13C]butyrate. $$$\alpha$$$kB and $$$\alpha$$$kV are promising alternatives for radical-free and metal-free translational clinical hyperpolarized MRI with high polarization and no need for radical removal via filtration.Acknowledgements
This study was supported by funding from the Swiss National Science Foundation (grant number PZ00P3_167871), the Emma Muschamp foundation, the Swiss Heart foundation and the Leenaards and Jeantet foundation. We also thank Da Silva Analina Raquel, Bressoud Valentine and Mitrea Stefanita-Octavian for their support with the in vivo experiments.References
- Ardenkjær-Larsen, J. H., Fridlund, B., Gram, A. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. PNAS. 2003;100(18):10158-10163.
- Lumata, L., Merritt, M. E., Kovacs, Z. Influence of deuteration in the glassing matrix on 13 C dynamic nuclear polarization. PCCP. 2013;15(19), 7032-7035.
- Ardenkjaer-Larsen, J. H. On the present and future of dissolution-DNP. JMR. 2016;264, 3-12.
- Eichhorn, T. R., Takado, Y., Salameh et al. Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. PNAS. 2013;110(45), 18064-18069.T.
- Bastiaansen, J. A., Yoshihara, H. A., Capozzi, A. et al. Probing cardiac metabolism by hyperpolarized 13 C MR using an exclusively endogenous substrate mixture and photo‐induced nonpersistent radicals. MRM. 2018;79(5), 2451-2459.
- Capozzi, A., Karlsson, M., Petersen J.R. et al. Liquid-state 13C Polarization of 30% through Photoinduced Nonpersistent Radicals. JPCC. 2018;122(13), 7432-7443.
- Marco-Rius, I., Cheng, T., Gaunt, A. P. et al. Photogenerated Radical in Phenylglyoxylic Acid for in Vivo Hyperpolarized 13C MR with Photosensitive Metabolic Substrates. JACS, 2018;140(43), 14455-14463.
- Capozzi, A., Patel, S., Gunnarsson, C. P. et al. Efficient Hyperpolarization of U‐13C‐Glucose Using Narrow‐Line UV‐Generated Labile Free Radicals. Angew Chem Int Ed Engl, 2019;58(5), 1334-1339.
- Mishkovsky, M., Anderson, B., Karlssonet, M. et al. Measuring glucose cerebral metabolism in the healthy mouse using hyperpolarized 13 C magnetic resonance. Scientific reports 7.1. 2017;7(1), 11719.
Figures