3018
Selective hyperpolarized 13C metabolites suppression from a liver-specific gadolinium contrast agent using an NMR-compatible bioreactor1University of California, San Francisco, San Francisco, San Francisco, CA, United States, 2Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China, 3Washington University, Seattle, WA, United States
Synopsis
This study demonstrated that hyperpolarized 13C metabolic signals arising from intracellular and extracellular 13C metabolites can be selectively suppressed by the intracellular liver-specific gadolinium contrast agent. This data further elucidates how targeted relaxation agents affect observed hyperpolarized 13C signals, expanding the way these agents can be used to distinguish between specific cell types in vivo.
abstract
Introduction:Hyperpolarized (HP) 13C MR is a powerful technology for monitoring real-time metabolism in tumors. Elevated production of [1-13C]lactate from [1-13C]pyruvate is near-universally observed in many tumors, including those in the liver1. Recently, the hepatocyte-specific gadolinium-based contrast agent gadoxetate has been proposed as a method to selectively suppress hyperpolarized signal arising from normal liver cells, while isolating the signals arising from tumor cells2. Although this strategy has been successfully implemented in vivo, little is known about the detailed interactions of hyperpolarized metabolites with intracellular and extracellular gadolinium. The aim of this study is to use cell culture and an NMR-compatible bioreactor to investigate the effects of intracellular gadoxetic acid on the observed signals arising from intracellular and extracellular 13C metabolites.Methods:Embryonic kidney HEK293 cells were stably transfected with human organic anion-transporting polypepetide 1B1(OATP1B1), which is the transporter that is chiefly responsible for importing gadoxetate into cells3,4. And HEK293 cells with empty vector (HEKEV) were used as a control. These cells were prepared for bioreactor NMR experiments by electrostatic encapsulation into alginate microspheres5. The cell-laden microspheres were maintained at physiological conditions by circulating DMEM media at 37℃ with continuing oxygen in the 5mm NMR tubes used in bioreactor. Experiments were conducted on a 500 MHz Varian Inova with a 5mm probe at 37℃. 7.5μL of [1-13C]pyruvic acid was hyperpolarized using a HyperSense DNP polarizer operating at 3T and subsequently dissolved and neutralized for a final concentration of 50mM. 900 uL of this solution was infused into the bioreactor system and NMR experiments were performed by application of a 30° degree pulse every 3s, for a total acquisition time of 300 s, using a sweep width of 100ppm. The peak intensity for each metabolite was computed at every time point. Metabolite signals were expressed as a fraction of the total observed HP carbon signal. Intracellular and extracellular lactate signals were separated based on chemical shift as described previously. In order to determine the effects of intracellular gadoxetate on the HP metabolite signal, HP 13C experiments were performed prior to and after infusion of 0.5 mM gadoxetate. Control experiments were performed using the same protocol with HEKEV cells. Gadopentetate dimeglumine, which is a strictly extracellular contrast agent was also used as a control to demonstrate the total reduction of lactate in OATP1B1 cells due to gadoxetate. Cell viability was dynamically monitored by measuring the peaks of nucleoside triose phosphate produced by the ATP molecule using 31P NMR. All image processing and analysis was performed using MestReNova and MATLAB.
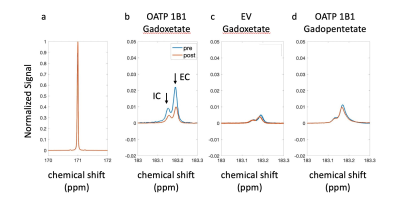
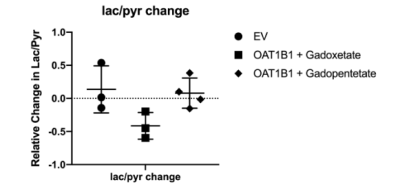
Results: The experimental setup is shown in Fig. 1. After infusion of HP 13C pyruvate, splitting of the lactate peak into two distinct resonances was observed, which has been previously ascribed to the intracellular and extracellular lactate pools, at approximately 183.14 ppm and 183.17 ppm, respectively6. In all experiments, extracellular lactate was higher than intracellular lactate, suggesting a rapid lactate export in these cell lines. Of the experiments performed with OATP1B1 cells and gadoxetate, two gave peaks that were separable and amenable to independent analysis. In these experiments, total production of intracellular and extracellular lactate was reduced by 24.77% and 33.53%, respectively (Fig.2 b). By contrast, in the HEK-EV group, the reduction of intracellular and extracellular lactate was little (Fig.2 c). The ratio of lactate to pyruvate was significantly lower in the gadoxetate group compared to HEKEV group (Fig.3). The fractional change in lactate/pyruvate ratio before and after administration of gadolinium or gadopentetate was calculated for each experiment based on the total area under the curve for each resonance. The relative change in lactate/pyruvate ratio observed in OATP1B1 cells with gadoxetate (-0.41 +/- 0.20) was significantly different than the change observed with OATB1B cells and gadopentetate (0.079+/-0.23, p = 0.03) and near-significantly different than the change observed after administration of gadoxetate to cells that do not express OATB1B ( 0.14 +/-0.36, p = 0.08) (Fig. 3). Discussion: Reduction of intracellular and extracellular hyperpolarized lactate levels were observed after giving gadoxetate in HEK296 cells that expressed OATP1B1 receptor but no such reduction was observed in cells not expressing the receptor. The extracellular contrast, gadopentetate, similarly did not affect the observed lactate production in these cells. Although gadoxetate is located inside the OATP1B1-expressing cells, it suppresses signals arising both intracellular and extracellular pools because the extracellular hyperpolarized lactate was exported from the (suppressed) intracellular pool. Meanwhile, when the strictly extracellular gadolinium agent (gadopentetate) was used in OATP1B1, total production of intracellular and extracellular lactate was only reduced by 7.6% and 5.4%, respectively (Fig.2 d). This result further confirmed that the decrease in intracellular and extracellular lactate was caused by gadoxetate residing in OATP1B1 cells. All results indicated that intracellular gadoxetic acid had an effect on the observed signals arising from intracellular and extracellular 13C metabolites.
Conclusion:Using a perfusion bioreactor system and isolated cells, we were able to observe the selective effect of the intracellular gadolinium agent, gadoxetate, on the hyperpolarized signal generated within cells. In the future, this system will enable better understanding of how targeted intracellular relaxation agents affect observed HP 13C signals, expanding the way these agents can be used to isolate specific cell types in vivo.
Acknowledgements
This study was funded by grant from National Institute for Biomedical Imaging and Bioengineering (R21EB023605) and HMTRC hyperpolarized resource center grant (NIBIB P41EB013598). Ting Sun is funded by China Scholarship Council (The State Scholarship Fund) as a visiting scholar at UCSF.References
1. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia (New York, NY) 2019;21(1):1-16.
2. Ohliger MA, von Morze C, Marco-Rius I, et al. Combining hyperpolarized (13) C MRI with a liver-specific gadolinium contrast agent for selective assessment of hepatocyte metabolism. Magnetic resonance in medicine 2017;77(6):2356-63.
3. Shu Y, Sheardown SA, Brown C, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. The Journal of clinical investigation 2007;117(5):1422-31.
4. Leonhardt M, Keiser M, Oswald S, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug metabolism and disposition: the biological fate of chemicals 2010;38(7):1024-8.
5. Keshari KR, Sriram R, Koelsch BL, et al. Hyperpolarized 13C-pyruvate magnetic resonance reveals rapid lactate export in metastatic renal cell carcinomas. Cancer research 2013;73(2):529-38.
6. Sriram R, Van Criekinge M, Hansen A, et al. Real-time measurement of hyperpolarized lactate production and efflux as a biomarker of tumor aggressiveness in an MR compatible 3D cell culture bioreactor. NMR in Biomedicine 2015;28(9):1141-9.
Figures