3015
Real-time Monitoring of in vivo free radical scavengers through hyperpolarized [1-13C] N-acetyl cysteine1National Cancer Institute, National Institutes of Health, Bethesda, MD, United States, 2Imaging Probe Development Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Rockville, MD, United States, 3National Institutes of Health, Bethesda, MD, United States, 4Department of Electrical Engineering, Technical University of Denmark, Lyngby, Denmark, 5NCI/RBB, National Institutes of Health, Bethesda, MD, United States
Synopsis
N-acetyl cysteine (NAC) is a widely used therapeutic and involved to stimulate glutathione synthesis. Glutathione elevates detoxification and works directly as a free radical scavenger. Here, we synthesized and demonstrated [1-13C]NAC as a promising novel probe for hyperpolarized 13C MRI methodologies, which has limitations in available number of probes. In vivo hyperpolarized NAC was broadly distributed throughout the body. The chemical conversions into products were observed in pancreatic tumor xenografts, Hs766t, and SU.86.86, with various conversion efficiencies depending on metabolic characteristics and status. Hyperpolarized NAC can provide insights into redox status, metabolic profile, and enzymatic activities.
Purpose
N-acetyl cysteine(NAC), the acetylated derivative of the amino acid L-cysteine, is a precursor of glutathione. NAC stimulates glutathione synthesis, since cysteine supply in glutathione appears to be the rate-limiting step in the glutathione synthesis.1 NAC also elevates glutathione-S-transferase activity, promotes liver detoxification, and also acts directly as a free radical scavenger. Therefore, it has been widely used as a beneficial antioxidant and potential therapeutic agent in the treatment of cancer, heart disease, HIV infection, and other diseases related to oxidative stress. For example, NAC is administered to treat acute acetaminophen overdose.1 Although N-acetyl cysteine is widely used in clinical practice, investigation to elucidate the real-time in vivo biodistribution and pharmacokinetics administered by intravenous infusion is was not possible to determine functions and to effectively design prodrugs of L-cysteine.2,3 Here, we synthesized and evaluated [1-13C]NAC as a promising candidate for in vivo hyperpolarized 13C MRI. We evaluated conditions for optimal level of polarization needed for in vivo imaging. The biodistribution of hyperpolarized [1-13C]NAC demonstrates that significant level of in vivo signals can be observed globally in vivo. Hyperpolarized [1-13C]NAC can potentially provide imaging assessment of monitoring antioxidant and enzymatic activities.Methods
Synthesis of [1-13C] N-acetyl cysteineAll commercially available reagents were used as received unless otherwise noted. [1-13C] L-cysteine and D2O were purchased from Cambridge Isotope Laboratories.
Hyperpolarized 13C MRI
35 ml of 3.2 M [1-13C]NAC with 17 mM Ox063 was hyperpolarized using the SPINlab (GE Healthcare) for 3-4 hours, and the scans were performed using the Philips Achieva 3T MRI. 13C two dimensional spectroscopic chemical shift images (CSIs) were acquired with a 28x 28 mm, a matrix size of 14 x 14, repetition time of 86 ms, and excitation pulse width a flip angle of 3o for the mouse head, and with a 32 x 32 mm, a matrix size of 16 x 16, repetition time of 85 ms, and excitation pulse with a flip angle of 10o for the mouse body. CSIs were acquired 30 seconds after the beginning of the hyperpolarized [1-13C]NAC injections.
Results and Discussion
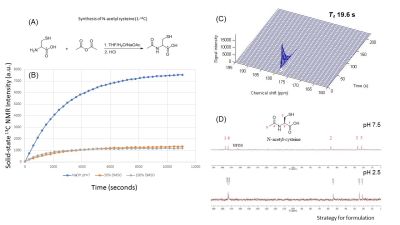
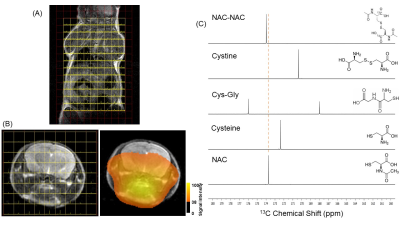
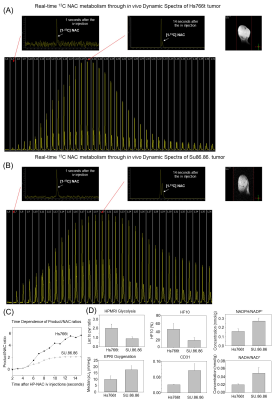
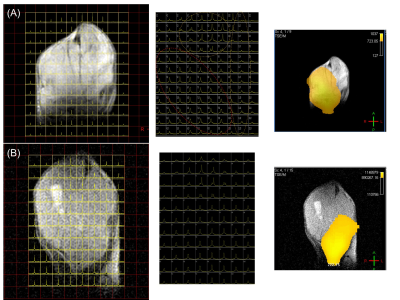
To formulate hyperpolarizing solutions, the synthesized [1-13C]NAC (Figure 1(A)) was dissolved using 5M NaOH until it reached a neutral pH = 7.5. This resulting solution of [1-13C]NAC became a homogenous self-glassing solution when frozen without utilizing conventional solvents, including glycerol or DMSO. This polarizing solution shows excellent polarization build-up, as shown in Figure 1(B) compared to mixtures with organic solvents. The decay dynamics of 13C MR signal of hyperpolarized [1-13C]NAC at 3T MRI determined the T1 relaxation time to be 19.6 seconds, as shown Figure 1(C). The polarizing solution of NAC was stable overtime as shown Figure 1(D). We conducted 13C two dimensional CSIs in both a healthy mouse body and head after iv injection of hyperpolarized [1-13C]NAC solution through a tail vein cannula as shown in Figure 2. These CSIs revealed that hyperpolarized [1-13C]NAC was globally distributed throughout the mouse body within 30 seconds after the injection of hyperpolarized solutions. Observed signals were higher in the liver, kidney, and heart region. On the other hand, the signals were relatively lower in the lung region, as shown in Figure 2(A). The biodistribution of hyperpolarized [1-13C]NAC in the normal mouse brain indicates that the NAC goes through the blood-brain barrier immediately and is also retained in the brain region with a detectable concentration using hyperpolarized 13C MRI methods, as shown in Figure 2(B). Metabolites of in vivo hyperpolarized [1-13C]NAC were not observed in the liver and kidney regions of these normal mice suggesting that the enzymatic conversion of NAC was below the detection level in the absence of any imposed oxidative stress focally or globally. The potential metabolites of hyperpolarized [1-13C]NAC were measured on 13C solution NMR spectroscopy as shown in Figure 2(C). Here, real-time dynamic 13C MR spectra of hyperpolarized [1-13C]NAC were acquired in the human pancreatic tumor xenografts of Hs766t and SU.86.86 in Figure 3. As shown in Figure 3(C), Hs766t and SU.86.86 exhibited distinct differences in the tumor microenvironment, physiology, and metabolic profiles. The differences in the chemical conversion efficiencies of [1-13C]NAC (Hs766t > SU.86.86) in the HP-13C dynamic spectra are attributed to these microenvironmental differences. Such observations were consistently observed in spatially resolved using 13C Chemical Shift Imaging as well as shown in Figure 4. Metabolomics approaches based on NMR and MS were conducted to reveal more detailed analysis of metabolites of hyperpolarized [1-13C]NAC in tumor xenografts. Using suitable animal disease models, and clinical/biological targets, hyperpolarized [1-13C]NAC can be used to probe the enzymatic activities or oxidative stress throughout the body and brain regions.Conclusions
The biodistribution of hyperpolarized [1-13C]NAC demonstrates that significant level of signals can be observed in the globally in a mouse, including the heart, liver, kidneys, brain, muscle, and lungs. Hyperpolarized [1-13C]NAC can be potentially be used for probing free radical scavengers and enzymatic activities, including acylases.Acknowledgements
This study was supported by intramural research program at NCI/NIH.References
[1] Meister A et al., Glutathione, Ann Rev Biochem. 1983;52:711-760.
[2] Yamauchi A et al., Tissue distribution of and species differences in deacetylation of N-acetyl-L-cysteine and immunohistochemical localization of acylase I in the primate kidney., J Pharm Pharmacol. 2002;54(2):205-212.
[3] Uttamsingh V et al., Immunohistochemical Localization of the Acylases that Catalyze the Deacetylation of N-Acetyl-l-cysteine and Haloalkene-Derived Mercapturates. 2000;28(6):625-632.
Figures