3012
Safety, Tolerability and Reproducibility of HP13C-MRI in Normal, Healthy Human Subjects
Robert Bok1, Hsin-Yu Chen1, Jeremy Gordon1, Adam Autry1, Shuyu Tang1, Brian Chung1, James Slater1, Peder E.Z. Larson1, Yan Li1, Mark van Criekinge1, Priscilla Chan1, Romelyn Delos Santos1, Evelyn Escobar1, Kimberly Okamoto1, Mary Frost1, John Kurhanewicz1, and Daniel B. Vigneron1
1Radiology & Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
1Radiology & Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
HP 13C-MRI with 13C-Pyruvate was performed in normal, healthy volunteers over four major body regions and organs, including head/brain, chest/heart, abdomen/liver, and pelvis/kidneys. Many individuals received multiple HP 13C injections and a variety of acquisition sequence. Pyruvate-to-lactate conversion rate measurements were reproducible across days and coil configurations. All injections and imaging were very well tolerated with no adverse effects observed in over 70 scans.
INTRODUCTION
The goal of this study was to conduct studies in normal, healthy volunteers to quantitatively assess normal values, develop techniques and obtain optimal scanning parameters on a 3 Tesla MRI using hyperpolarized (HP) 13C-pyruvate. Three classes of experiments were performed: technique validation, RF coil performance assessments, and normal values measurements. Here we report our experience to date regarding safety, tolerability and reproducibility for imaging of the head, chest, abdomen and pelvis with HP 13C-MRI.METHODS
Human Subjects: These volunteer studies were conducted under the aegis of a UCSF IRB-approved protocol. A total of 15 adults (5 females, 10 males) were interviewed to confirm the absence of any significant current or past health problems (i.e., no history of cancer, kidney disease, heart disease, diabetes or other major medical issues) and consented for the HP 13C-MRI Study. On day of study, subjects had vital signs and weight measured. A peripheral intravenous catheter was placed and subjects were positioned inside a 3T MRI scanner with appropriate 1H and 13C coils for the body section of interest to be scanned.Sample Preparation: The sample preparation was carried out in collaboration with UCSF Radiopharmacy. Each sample consisted of a mixture of GMP grade [1-13C]- or [2-13C]pyruvic acid (14.2 M; MilliporeSigma Isotec) and 15 mM Electron Paramagnetic Agent (EPA), (AH111501, GE Healthcare). Samples were prepared the same morning of each study and inserted under aseptic conditions into pharmacy kits from GE Healthcare.
Polarization: A SPINlab polarizer (GE Healthcare) was used to polarize [1-13C]pyruvate at 5T and 0.8 K. The sample was irradiated with microwave at fMW= 140.02 GHz for 2 hours, achieving a 13C polarization of ~40% in the liquid state.
Pre-injection Quality Check & Injection: Following dissolution, the EPA was filtered mechanically and quality control carried out prior to injection: pH, pyruvate and EPA concentrations, polarization, and temperature were measured and compared to ranges of acceptability. In parallel, integrity of the 0.2 μm sterile filter was tested following manufacturer specifications to a pressure-stress of 50 psi. After release by the pharmacist, ~250 mM pyruvate was injected at a dose of 0.43 mL/kg and a rate of 5 mL/s, followed by a 20 mL saline flush.
Acquisition: All experiments were performed on a 3T MR scanner (MR750, GE Healthcare) with multi-nuclear excitation and 32-channel reception capability. Brain studies were performed using a birdcage for RF excitation and either a 24-channel or 32-channel coil for data acquisition. All other studies utilized a clamshell volume coil for RF transmit and either an 8- or 16-channel coil for receive. Images of pyruvate, lactate, bicarbonate or alanine (dependent on organ of interest) were acquired using either spectroscopic imaging or rapid imaging approaches (EPI/Spiral1,2, SSFP3, EPSI4) with a 2-3 s temporal resolution. Scan parameters for a standard acquisition and coil configurations are provided in Table 1.
Reconstruction and Analysis: Multi-channel 13C data was combined using coil sensitivity maps estimate from the pyruvate signal5. An inputless two-site exchange model6 was applied to quantitatively characterize pyruvate-to-lactate conversion rate, kPL.
RESULTS AND DISCUSSION
We have conducted HP 13C-MRI studies in 15 separate normal, healthy individuals to date, performing a total of 71 HP 13C-agent injections. While both [1-13C]-Pyruvate and [2-13C]-pyruvate7 agents were employed, the majority of the studies have been with the C1 compound (61 injections/scans) and only these data are detailed here. The body regions/organs studied included head/brain, chest/heart, abdomen/liver and pelvis/kidney. Several of these individuals received 2 injections 15-60 min apart and also over multiple different sessions. Two subjects have received over a dozen injections: one has received 14 injections over 10 sessions; another has received 19 injections over 12 sessions. No adverse effects have been observed in either of these individuals, nor in any of the others studied to date. Only minor, transient alterations in sensations have been noted by subjects, such as disturbances in taste (usually “metallic” in character) lasting only a few seconds or a sensation of cold or warmth in the arms or upper body (again, lasting only a few seconds).Conversion rates of pyruvate-to-lactate (kPL) or pyruvate-to-bicarbonate (kPB), and/or ratios of Lactate/Pyruvate (Lac/Pyr) and Bicarbonate/Pyruvate (Bic/Pyr) ratios were estimated (Figure 1). From available data in the brain (Figure 2, Table 3), test-retest reproducibility was observed in two brain subjects between studies at different times (same day) and different days using various C-13 transceiver setups.
CONCLUSIONS
This study employed HP 13C-MRI to interrogate typical metabolism levels of pyruvate-to-lactate and pyruvate-to-bicarbonate conversion rates in the major body regions and organs in normal, healthy adults. No adverse events were observed in any of the subjects. These results add to earlier phase 1 studies conducted in cancer patients8 and other smaller cohorts of healthy volunteers9,10.11 which demonstrated the safety of this metabolic imaging modality on selected organs. In summary, HP 13C-MRI with 13C-pyruvate appears to be extremely safe and well tolerated over all major body regions and shows reproducibility in the most studied normal organ to date. While still preliminary, the results from this project are promising and should provide critical normative data for on-going and future studies of disease with HP 13C-MRI.Acknowledgements
This work was funded by the NIH (U01EB026412, P41EB013598) and the UCSF NICO project.References
- Gordon JW, Vigneron DB, Larson PEZ. Development of a symmetric echo planar imaging framework for clinical translation of rapid dynamic hyperpolarized 13C imaging. Magn. Reson. Med. 2017;77(2):826-832.
- Lau AZ, Chen AP, Hurd RE, Cunningham CH. Spectral-spatial excitation for rapid imaging of DNP compounds. NMR in Biomed. 2011;24(8):988-996.
- Milshteyn E, von Morze C, Reed GD, et al. Development of high resolution 3D hyperpolarized carbon-13 MR molecular imaging techniques. Magn. Reson. Imaging 2017;38:152-162.
- Chen H-Y, Larson PEZ, Gordon JW, et al. Technique development of 3D dynamic CS-EPSI for hyperpolarized 13C pyruvate MR molecular imaging of human prostate cancer. Magn. Reson. Med. 2018;80(5):2062-2072.
- Zhu Z, Zhu X, Ohliger MA, et al. Coil combination methods for multi-channel hyperpolarized 13C imaging data from human studies. J. Magn. Reson. 2019;301:73-79.
- Larson PEZ, Chen HY, Gordon JW, et al. Investigation of analysis methods for hyperpolarized13C-pyruvate metabolic MRI in prostate cancer patients. NMR in biomedicine 2018;31(11): e3997.
- Chung BT, Chen HY, Gordon J, et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J. Magn. Reson. 2019;309:106617.
- Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic Imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl. Med. 2013;5(198):198ra108.
- Cunningham CH, Lau JY, Chen AP, et al. Metabolic MRI of the human heart: initial experience. Circulation Res. 2016;119(11):1177-1182.
- Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. NeuroImage 2019;189:171-179.
- Lee CY, Soliman H, Geraghty BJ, et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. NeuroImage 2020;204:116202.
Figures
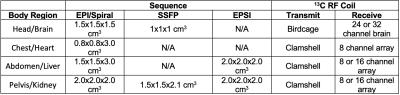
Table 1. Sequence and Coil Configurations Used
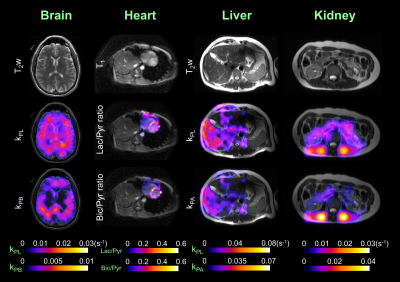
Figure 1. Examples of the four major body regions and associated organs studied in healthy adult volunteers by HP 13C-MRI with [1-13C]pyruvate.
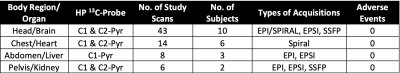
Table 2. Summary of Healthy Subjects HP 13C-MRI Scans To Date
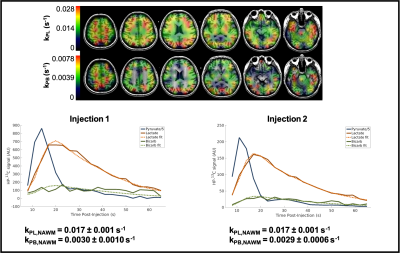
Figure 2. Depiction of reproducibility of HP 13C-MRI with [1-13C]Pyruvate in the brain of a healthy volunteer (Subject 1) conducted over two scans, separated by ~1hr . Quantitative values of kPLdeterminations are listed in Table 3. Subject 2 parameters show the reproducibility over several separate scan sessions and over different receiver coils.
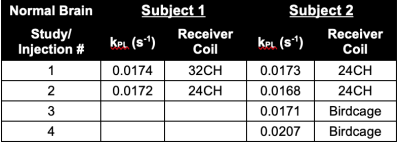
Table 3. Examples of Test-Retest Results from HP 13C-MRI Brain Studies