3010
Differentiating Between DBD and Normotensive Syngeneic Grafts in a Rat Lung Transplant Model Using HP [1-13C] Pyruvate MRI1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Surgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Our previous study in a rat lung transplant model using HP [1-13C]-pyruvate MRI demonstrated that an increased lactate-to-pyruvate ratio (LtP) precedes lung rejection. However, most lungs in a clinical setting are obtained after donor brain death (DBD), which is associated with increased ischemia-reperfusion injury and risk of acute lung rejection. In this study, we transplanted DBD-lungs into syngeneic rats and compared the metabolism of DBD-donor lungs to our previous normotensive-donor model. The LtP of the DBD-graft is approximately two-fold higher than that of the other lungs, suggesting that HP [1-13C]-pyruvate imaging can be used to identify grafts with ischemia-reperfusion injury.
Introduction
In a previous study of syngeneic and allogeneic grafts in a rat model using hyperpolarized (HP) [1-13C] pyruvate MRI, we found that lung rejection is preceded by an increased lactate-to-pyruvate ratio (LtP) in the allograft compared to an isograft1. While that study was conducted using normotensive donors, most lungs in a clinical setting are obtained after donor brain death (DBD), which is associated with increased ischemia-reperfusion injury and a concomitant increase in the risk of acute lung rejection. In order to determined whether our previous findings help in a model that more closely recapitulates the clinical reality, in this study we transplanted lungs obtained after DBD into healthy syngeneic rats and compared the graft performance and metabolic profile of DBD donor lungs to our previous donor model of normotensive brain death.Methods
Left lung isografts were transplanted from inbred Dark Agouti to Dark Agouti rats. N=3 normotensive and n = 3 DBD donors were used. DBD animals were placed under anesthesia and mechanically ventilated during the DBD procedure. Induction of donor brain death was performed as previously published2. Briefly, a hole was drilled through the skull to expose the dura; a 4F Fogarty catheter was inserted into the extradural space and inflated for 30 minutes to induce brain death, which was confirmed via the cessation of brain electrical activity as measured using an EEG. Orthotopic transplant surgery was performed as previously described, with a single-suture bronchial anastomosis technique and a proximal cuffing approach for vascular anastomoses3. Total surgery duration was about 3.5h, with approximate ischemia time of 60 minutes. HP [1-13C]-pyruvate MR imaging was performed on days 3 and 7. Animals were imaged while supine in a 4.7T magnet (Varian Inc.). HP [1-13C]-pyruvate (28.6mg, 15mM OX063, 1.5mM Dotarem Gd) was polarized using a HyperSense DNP polarizer, and ~1.2mL (4mL/kg, 80mM) of HP agent was injected via the tail vein over 6s. HP [1-13C] pyruvate chemical shift imaging (CSI) was performed using a 2D slice selective phase-encoded FID-CSI sequence (TR/TE= 35.7/0.35ms, α= 9°, FOV= 45x45x8mm3), as previously reported4. Spectra were reconstructed, processed and analyzed using custom MATLAB scripts. A subset of animals were sacrificed and their lungs fixed for histological processing.Results
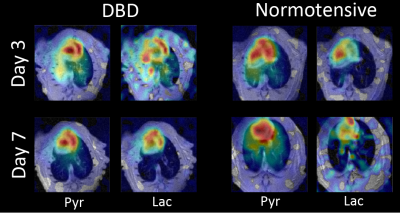
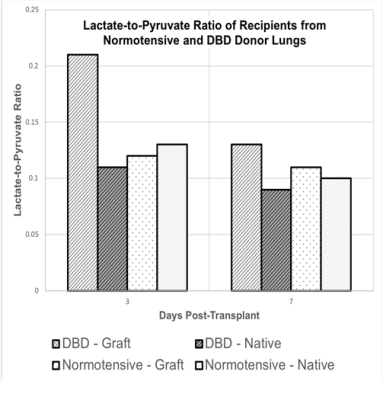
Figure 1 shows representative pyruvate and lactate maps of the DBD and normotensive rat lungs on days 3 and 7. The DBD graft shows much higher pyruvate and lactate signals on day 3. This is most likely due to hyperperfusion as well as increased inflammation resulting from the increased pre-inflammatory state of the DBD donor lungs. In comparison, the normotensive cohort shows minimal differences between the native and transplanted (left) lungs. All four lungs show similarly low levels of lactate by day 7. Figure 2 shows the quantitative summary of our findings. The native lungs in normotensive and DBD cohorts show a mean day 3 lactate-to-pyruvate ratio (LtP) of 0.13±0.03 and 0.11±0.03, respectively. The respective LtPs decrease slightly to 0.10±0.02 and 0.09±0.03 on day 7, most likely as pyruvate hyperperfusion decreases with inflammation subsidence. The LtP of the normotensive graft was 0.12±0.03 and 0.11±0.03 on days 3 and 7, respectively, suggesting that there were no differences between the native lung and the graft in this cohort. The LtP of the DBD graft was 0.21±0.05 and 0.13±0.04 on days 3 and 7, respectively.Conclusions
The lactate-to-pyruvate ratio of the DBD graft is approximately two-fold higher than that of the native lungs in both cohorts and of the normotensive graft, suggesting that HP [1-13C]-pyruvate imaging can be used to identify grafts with ischemia-reperfusion injury. The next step would be to image DBD grafts in immunosuppressed allogeneic recipients to mimic the clinical setting.Acknowledgements
References
[1] S Siddiqui, et al. NMR in Biomedicine. March 2019
[2] Pratschke J,et al. Transplantation 2000;69:427–30.
[3] Habertheuer, et al. Journal of Surgical Research, 2013.
[4] Pourfathi, et al. ISMRM, Singapore, 2016.
Figures