3005
Hyperpolarized MRI Evaluation of Streptozotocin Injection in the Fasted or Postprandial State: A Metabolic and Functional Study of Diabetes1University of Oxford, Oxford, United Kingdom
Synopsis
Heart failure in diabetes is the leading cause of mortality in patients. Many studies show cardiac dysfunction in the STZ-induced diabetes model, however variability in cardiac metabolism and function is observed due to differences in the method of model induction. This study investigated both a high and a low dose STZ-induced diabetic model, with STZ injected in both the fasted and postprandial states. Different metabolic and functional severities were observed; with the high-dose STZ-induced diabetic model injected in the fasted state resulting in the most severe form of diabetes with remodelling of both cardiac function and metabolism.
Introduction
Type-1 diabetes patients are insulin deficient resulting in hyperglycaemia. Diabetes increases the incidence of myocardial infarction and heart failure, which are the leading cause of mortality in diabetic patients [1]. Streptozotocin (STZ) injection in rodents destroys the pancreatic β-cells, thereby inducing a model of type-1 diabetes. Many studies confirm cardiac dysfunction in the STZ induced rodent model [2,3], however, the metabolic state during the STZ injection often varies across studies leading to variable results. In this work, we have characterized key in vivo metabolic and functional differences following STZ injection in both the fasted and postprandial state to allow for a better selection of an appropriate STZ induced diabetes model for future studies.Methods
28 Male Wistar rats (~200g) were split into a control group (CTR; n=8), a low dose streptozotocin (STZ)-induced diabetic group injected in the postprandial state (LowD(Fed), 25 mg/kg; n=4), a low dose STZ-induced diabetic group injected in the fasted state (LowD(fasted), 25 mg/kg; n=4), a high dose STZ-induced diabetic group injected in the postprandial state (HighD(Fed), 55 mg/kg; n=4), and a high dose STZ-induced diabetic group injected in the fasted state (HighD(Fasted), 55 mg/kg; n=8). Two weeks post STZ injection, ECG-gated 13C MR pulse-acquire cardiac spectra were acquired over 60s following injection of hyperpolarized [1-13C]pyruvate (repetition time 1s; excitation flip angle 15°; sweep width 13,021Hz; acquired points 2,048; frequency centred on the C1 pyruvate resonance). Spectra were summed over 30s from the first appearance of pyruvate and analysed with JMRUI for metabolic assessment of the heart [4]. In addition, eight-ten short-axis slices (slice thickness:1.6 mm, matrix size:128×128, TE/TR:1.67/4.6ms, flip angle:15°, number of averages:4) were acquired with a CINE-FLASH sequence and analysed with ImageJ for assessment of cardiac function.Results
The control animals gained weight over the course of the experiments (106.9±24.6g), the two low-dose STZ groups also gained weight (Fasted:75.8±26.0g, Postprandial:89.5±17.1g). The high-dose groups gained minimal weight (Fasted:4±14.4g, Postprandial:8.3±37.2g) over the experimental course (Fig. 1A). Blood glucose levels were significantly elevated in all STZ groups compared to controls, even though the average blood glucose of the low-dose STZ (Fasted:7.8±0.6mmol/l, Postprandial:7.7±0.8mmol/l) did not reach levels as high as the high-dose STZ groups (Fasted:19.4±6.7mmol/l, Postprandial:11.8±3.7mmol/l, Fig. 1BC).PDH-flux (bicarbonate/pyruvate) was significantly reduced in the fasted high-dose group by 64% compared to the control group (p=0.02). The postprandial high-dose group had a similar (66%) reduction in PDH-flux, however, it failed to reach statistical significance (p=0.06, Fig.2A). Lactate was unchanged across all groups (Fig.2B), whilst alanine was significantly reduced by 91% (p=0.006) in the postprandial high-dose group and by 57% in the fasted high-dose group compared to the control group (p=0.04, Fig.2C).
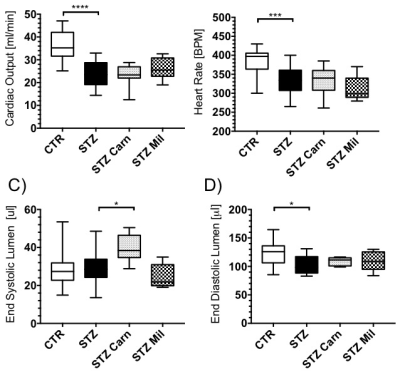
Cardiac output was significantly reduced by 28% in the fasted high-dose STZ group compared to the control group (p=0.017, Fig.3A). However, cardiac output was unchanged in all other STZ groups. Heart rate remained the same across all groups (Fig.3B). End Systolic lumen was reduced by 56% in the postprandial high-dose STZ group (p=0.008) and by 43% in the postprandial low-dose STZ group (p=0.049) compared to the control group (Fig.3C). End diastolic lumen showed a 21% decrease in the postprandial high-dose STZ group compared to the control group (p=0.045), whilst the other STZ groups showed no differences in EDL (Fig.3D).
Discussion and Conclusion
This study shows characterization of in vivo metabolism and function in different STZ induced diabetic rat models with metabolic remodelling observed in both of the high-dose STZ groups studied. However, only the fasted high-dose STZ group showed a significant reduction in PDH-flux, which is generally reduced in diabetes [5]. The fasted high-dose group also displayed reduced cardiac output. Therefore, a high-dose of STZ (55mg/kg) induced during the fasted state results in the most severe form of diabetes. No measurable change in in vivo metabolism was observed in either of the two low-dose STZ groups, despite mildly elevated glucose levels. This study therefore shows different metabolic and functional severity of STZ-induced diabetes, which can be varied with both STZ dose and either feeding or fasting prior to the STZ injection.Acknowledgements
We would like to thank Dr. Michael Pavlides, Stefan Neubauer, and Leanne Hodson for their supervision and their expertise metabolism and cardiac and liver MR imaging. We would also like to thank Annie Morsing, Novo Nordisk A/S for her guidance and mentorship.References
[1] T. J. Secrest, Aaron M. ; Washington, Raynard E.; Orchard, Mortality in Type 1 Diabetes. Diabetes in America, 2014.
[2] Heiko Bugger and E. Dale Abel. “Molecular mechanisms of diabetic cardiomyopathy”. In: Diabetologia, 2014.
[3] P.C. Huang "Cellular apoptosis and cardiac dysfunction in STZ-induced diabetic rats attenuated by anthocyanins via activation of IGFI-R/PI3K/Akt survival signaling." Environ Toxicol. 2017
[4] L. Vanhamme, A. Van Den Boogaart, and S. Van Huffel, “Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge,” J. Magn. Reson., 1997.
[5] AM Seymour, JC Chatham. "The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity". J. Mol. Cell Cardiol. 1997. [
[6] BK Kundu et al. ”Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis”. Cardiology. 2015.
Figures