3004
Boosting the sensitivity of MRI: 10,000 Tesla equivalent water proton signal1Department of Health Technology, Technical University of Denmark, Kongens Lyngby, Denmark
Synopsis
In recent years, hyperpolarization of water protons via dissolution Dynamic Nuclear Polarization has attracted increasing interest in the magnetic resonance community. Hyperpolarized water may provide an alternative to Gd-based contrast agents, and it may report on chemical and biochemical reactions, and proton exchange. However, hyperpolarizing water protons is challenging, since the presence of radicals is the main source of relaxation during dissolution and transfer to the MRI system. In this work, we report water signals otherwise requiring >10,000 T at room temperature by employing UV-generated labile radicals, opening up for novel MRI applications.
Introduction:
Since its birth, water protons have played a crucial role for Nuclear Magnetic Resonance (NMR). In particular, Magnetic Resonance Imaging (MRI) exploits the magnetism of the water protons of the human body to non-invasively visualize organs and tissues.1 Hyperpolarization (HP) via dissolution Dynamic Nuclear Polarization (dDNP) was introduced in 2003 by Ardenkjær-Larsen and co-workers,2 and it has become the most widespread and versatile hyperpolarization method to overcome the low sensitivity of NMR in the liquid state. Water protons have been efficiently polarized (30 – 40 %) in the solid state already at traditional dDNP conditions (3.35 T and 1.2 K),3 and close to unity polarization has been achieved by doubling the magnetic field.4 Nevertheless, preserving this fabulous spin order in the liquid-state is far from trivial. Water as such, characterized by a T1 of approx. 3.5 s,5 is not a molecule suitable for dDNP. UV-induced labile radicals have been employed to efficiently hyperpolarize 13C and other low-gamma nuclei by dDNP.6 UV-irradiation of a frozen solution containing a fraction of pyruvic acid (PYR) or PYR derivatives, generate radicals that are stable below 190 K.7 These radicals recombine into diamagnetic species during the dissolution, leaving a radical-free solution displaying long relaxation times suitable for MRI applications. Taking advantage of this unique property and optimizing the radical precursor, we have established a robust method to efficiently hyperpolarize water protons in the solid state and minimize polarization losses during and after dissolution.Methods:
Two UV-radical precursors were considered: natural abundance pyruvic acid (PYR) and [2-13C]pyruvic acid (2CPYR). Three mixtures were prepared: PYR:glycerol-d8:H2O 2:3:5 (v/v/v) (PYR sample), 2CPYR:glycerol-d8:H2O 2:3:5 (v/v/v) (2CPYR sample), and 50 mM TEMPOL in glycerol-d8:H2O 5:5 (v/v) (TEMPOL sample). Eight 6.0 ± 0.5 μL beads were transferred inside a synthetic quartz Dewar (Miniscope MS 5000 ESR, Magnettech, Berlin, Germany) filled with liquid nitrogen for UV irradiation (Dymax BlueWave 75, Torrington, CT USA). DNP and LOD ESR measurements were performed on a homebuilt dDNP polarizer working at 1.10 ± 0.05 K and 6.7 T. Microwaves were delivered from a 94 GHz solid-state source (Quinstar, USA) coupled to a 200X2R4 frequency doubler (VDI, Charlottesville, VA, USA). All LOD ESR acquisitions were obtained by means of a homemade spectrometer. After dissolution and transfer (2 s) the liquid state relaxation was monitored in a 9.4 T high resolution vertical NMR magnet (Varian, USA).Results:
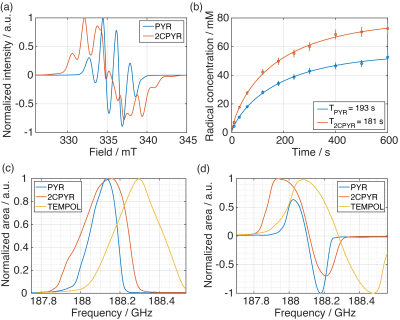
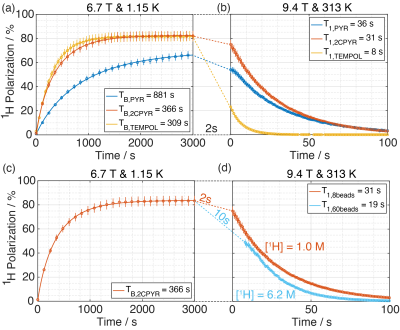
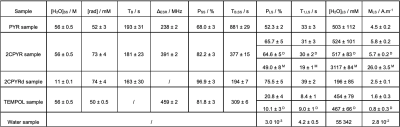
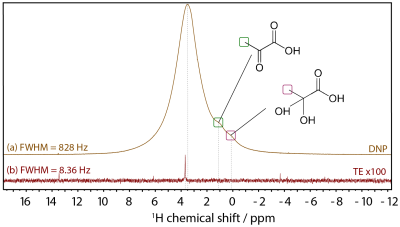
Figure 1 shows the X-band sweep spectra of PYR and 2CPYR samples (panel a) and their radical generation time course (panel b). In panel c is shown the LOD ESR spectra of PYR, 2CPYR and TEMPOL samples, together with their proton DNP sweep spectra shown in panel d. In Figure 2 we compare the polarization build-up (left panels) and consequent liquid state relaxation (right panels) of all samples. All numerical data relative to ESR spectra, 1H polarizations, and time constants are summarized in Table 1. Comparison between the thermal and enhanced signals is shown in Figure 3.Discussion:
The results show that solid-state DNP and build-up time constants for the UV-induced radicals are comparable to what has been achieved using the best stable, polarizing agents for protons, i.e. nitroxides. The solid-state polarization, using 2CPYR as radical precursor had the advantage of working with a radical specie characterized by a broader ESR spectrum than PYR. Moreover, the radical yield in the 2CPYR sample was also higher. Consequently, the larger amount of radical compared to the TEMPOL sample compensated for the narrower ESR spectrum achieving very similar solid-state DNP performance. Concerning dissolution and transfer of the HP solution, the results show that using UV-induced radicals leads to a polarization loss of only 21 % vs. 75 % for the TEMPOL sample. Besides, the absence of radical species in the liquid sample increases the liquid-state proton T1 from 8 s for TEMPOL sample up to 31 s for 2CPYR sample, making the implementation in a clinical environment more friendly. Finally, we observe that increasing the proton concentration still offers nearly 50 % proton polarization in the liquid state, corresponding to a magnetization otherwise requiring >10,000 T at room temperature.Conclusion:
We demonstrate a novel method for hyperpolarizing water protons that provides unprecedented nuclear spin magnetization. Our work pushes hyperpolarization of water close to the theoretical limit and opens up for a range of exciting applications in medicine, biology and chemistry. In particular, a proton magnetization equivalent to pure water in a >10,000 T magnetic field opens up for superior perfusion and angiographic imaging applications.Acknowledgements
The authors thank Magnus Karlsson and Mathilde H. Lerche for helpful discussions and advice, as well as Jonas Milani and Jan Kilund for building the magnetic tunnel and technical support. This work received funding from the Danish National Research Foundation (DNRF124); the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 713683 (COFUND fellows DTU).References
1 Lauterbur, P. C. Image Formation by Induced Local Interactions - Examples Employing Nuclear Magnetic-Resonance. Nature 242, 190-191, doi: 10.1038/242190a0 (1973).
2 Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. P Natl Acad Sci USA 100, 10158-10163, doi: 10.1073/pnas.1733835100 (2003).
3 Kurdzesau, F. et al. Dynamic nuclear polarization of small labelled molecules in frozen water-alcohol solutions. J Phys D Appl Phys 41, 155506 155501-155510, doi: 10.1088/0022-3727/41/15/155506 (2008).
4 Lipso, K. W., Bowen, S., Rybalko, O. & Ardenkjaer-Larsen, J. H. Large dose hyperpolarized water with dissolution-DNP at high magnetic field. J Magn Reson 274, 65-72, doi:10.1016/j.jmr.2016.11.008 (2017).
5 Anderson, W. A. & Arnold, J. T. Proton Relaxation Times in H2o - D2o Mixtures. Phys Rev 101, 511-512, doi: 10.1103/PhysRev.101.511 (1956).
6 Bastiaansen, J. A. M. et al. Probing cardiac metabolism by hyperpolarized 13C MR using an exclusively endogenous substrate mixture and photo-induced nonpersistent radicals. Magnet Reson Med 79, 2451-2459, doi:10.1002/mrm.27122 (2018).
7 Capozzi, A., Cheng, T., Boero, G., Roussel, C. & Comment, A. Thermal annihilation of photo-induced radicals following dynamic nuclear polarization to produce transportable frozen hyperpolarized 13C-substrates. Nat Commun 8, 15757, doi:10.1038/ncomms15757 (2017).
Figures