3001
Hyperpolarized [1-13C] Lactate as an Agent for Measuring Pyruvate Carboxylase Activity1AIRC, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 3Electrical Engineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
[1-13C] lactate was studied as hyperpolarized substrate to measure hepatic pyruvate carboxylase activity in vivo with fed and fasted conditions. Besides pyruvate, alanine and bicarbonate, 13C-aspartate could be detected from hyperpolarized lactate. Although the intracellular pyruvate pool size is tightly regulated, we found that lactate conversion to pyruvate and alanine is not saturated when we increased the concentration of hyperpolarized lactate from 30 to 60 mM, whereas the bicarbonate production was saturated. This study demonstrates the utility of hyperpolarized lactate to detect pyruvate carboxylase activity in vivo, and suggests that the metabolite ratio analysis should consider saturable enzyme activities.
Introduction
Pyruvate can be converted in the mitochondria either by pyruvate carboxylase (PC) or the pyruvate dehydrogenase (PDH) complex. PC catalyzes pyruvate to oxaloacetate (1). PC is the major pace-determining anaplerotic enzyme, which replenish the TCA cycle intermediates for gluconeogenesis, lipogenesis and amino acid synthesis (2). PC controls the balance between anaplerosis and cataplerosis. It is inappropriately increased in pathologies characterized by higher gluconeogenesis such as non-alcoholic fatty liver disease, diabetes and cancer (3). Dynamic nuclear polarization (DNP) of [1-13C] pyruvate allows real-time observation of cellular metabolism and in vivo enzyme activities by detecting the metabolic products such as lactate, alanine, and HCO3– (bicarbonate) (4, 5). In particular, HCO3– can be produced from both PDH and PC pathways, and previous studies showed limitation and controversy of interpreting HCO3– production from hyperpolarized [1-13C] pyruvate as PC activity (6-8). Lee, et al. demonstrated more direct measure of hepatic pyruvate carboxylation by detecting aspartate, malate and fumarate from hyperpolarized [1-13C] pyruvate at a higher magnetic field (7T) to take advantages of increase chemical shift dispersion (9). At 3T, however, the PC-specific products are difficult to resolve and often dominated by the large pyruvate and pyruvate hydrate peaks. Lactate is the key metabolite the Cori cycle and also the major substrate for liver gluconeogenesis (10). The control strength of PC for lactate gluconeogenesis is much greater for PC than for other enzymes of the pathway, making lactate a good probe to study PC activity in the liver (11). HP [1-13C] lactate is also shown to be effective for in vivo metabolism study (12, 13). Moreover, due to the tightly regulated pyruvate pool size, we expect to have much smaller pyruvate and pyruvate hydrate peaks from hyperpolarized [1-13C] lactate, allowing to resolve aspartate, malate, and fumarate resonances. Therefore, we hypothesized that hyperpolarized [1-13C] lactate can be used to detect PC metabolism at 3T.Methods
[1-13C] sodium lactate was purchased from Cambridge Isotope Labratories. Inc. GE SPINlab polarizer was used for polarizing [1-13C] sodium lactate. In vivo animal MR spectroscopy was performed at a clinical 3T MR scanner (GE Discovery 750W). 2.1M of [1-13C] sodium lactate was prepared in 4:1 w/w water:glycerol mix with 15mM OX063 as previously described [11]. Healthy male Wistar rats (200-400g) were prepared for in vivo experiments. A custom-built 13C radiofrequency surface coil (single loop, Ø = 28mm) was placed on top of the liver area for both excitation and data acquisition. Each rats were injected twice intravenously: one with 30-mM and one with 60-mM hyperpolarized [1-13C] lactate (0.375 or 0.75 mmol/kg body weight, up to 4.0 mL, injection rate = 0.25 mL/s). Data was acquired using a dynamic pulse-and-acquire spectroscopy sequence (TR = 3 sec, 32-msec 10o hard pulse, scan time = 4 min) simultaneously with the injection.Results and Discussion
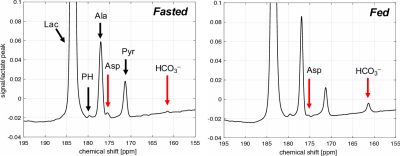
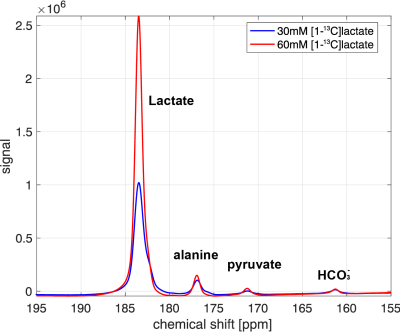
Fig.1 shows the time-averaged 13C spectra acquired after an injection of hyperpolarized [1-13C] lactate in a fasted (left) and fed (right) rat liver in vivo. [1-13C] aspartate peak was clearly detected from all the fasted rats while [13C] HCO3– peak was just above or at the noise level, suggesting a higher PC activity and lower PDH activity. On the contrary, the fed rats produced more HCO3– and less aspartate, indicating that PDH activity dominates PC. Fig.2 compares the time-averaged 13C spectra acquired after an injection of 60-mM (red) and 30-mM (blue) hyperpolarized [1-13C] lactate from the same rat (fed condition). Pyruvate and alanine production were proportional to the lactate concentration the amount of HCO3– production was comparable from the two injections probably due to the saturated PDH activity in the liver with 30-mM lactate injection (n = 3). We plan to repeat the experiments in fasted rats to investigate the saturable condition for PC.Conclusion
Hyperpolarized [1-13C] lactate was used to trace the 13C flux in the liver into aspartate through PC pathway and HCO3– through PDH pathway. The results suggest that hyperpolarized [1-13C] lactate is an improved method for PC and PDH activity detection than [1-13C] pyruvate.Acknowledgements
The Texas Institute of Brain Injury and Repair; The Mobility Foundation; National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award.References
1. Utter MF, Keech DB. Formation of oxaloacetate from pyruvate and carbon dioxide. J Biol Chem. 1960;235:Pc17-8.
2. Jitrapakdee S, Vidal-Puig A, Wallace JC. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci. 2006;63(7-8):843-54.
3. Lao-On U, Attwood PV, Jitrapakdee S. Roles of pyruvate carboxylase in human diseases: from diabetes to cancers and infection. J Mol Med (Berl). 2018;96(3-4):237-47.
4. Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158-63.
5. Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66(22):10855-60.
6. Merritt ME, Harrison C, Sherry AD, Malloy CR, Burgess SC. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc Natl Acad Sci U S A. 2011;108(47):19084-9.
7. Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007;104(50):19773-7.
8. Jin ES, Moreno KX, Wang JX, Fidelino L, Merritt ME, Sherry AD, et al. Metabolism of hyperpolarized [1-(13)C]pyruvate through alternate pathways in rat liver. NMR Biomed. 2016;29(4):466-74.
9. Lee P, Leong W, Tan T, Lim M, Han W, Radda GK. In vivo hyperpolarized carbon-13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin-resistant mouse model. Hepatology. 2013;57(2):515-24.
10. Hoffer LJ. Cori cycle contribution to plasma glucose appearance in man. JPEN J Parenter Enteral Nutr. 1990;14(6):646-8.
11. Wang D, Yang H, De Braganca KC, Lu J, Yu Shih L, Briones P, et al. The molecular basis of pyruvate carboxylase deficiency: mosaicism correlates with prolonged survival. Mol Genet Metab. 2008;95(1-2):31-8.
12. Mayer D, Yen YF, Josan S, Park JM, Pfefferbaum A, Hurd RE, et al. Application of hyperpolarized [1-(1)(3)C]lactate for the in vivo investigation of cardiac metabolism. NMR Biomed. 2012;25(10):1119-24.
13. Park JM, Josan S, Mayer D, Hurd RE, Chung Y, Bendahan D, et al. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol. 2015;218(Pt 20):3308-18.
Figures