3000
Effects of Anaerobic Exercise in Skeletal Muscle Measured by Hyperpolarized [1-13C]Pyruvate in Humans1Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Electrical Engineering, University of Texas Dallas, Richardson, TX, United States, 4GE Healthcare, GE Healthcare, Dallas, TX, United States
Synopsis
Direct studies of lactate and pyruvate metabolism by biopsy or vascular cannulation during or after exercise is undesirable. The fraction of pyruvate that is oxidized to CO2 vs. reduced to lactate in skeletal muscle was studied in healthy human subjects using hyperpolarized [1-13C]pyruvate at rest and after toe-lift exercise. There was a 2.5x fold increase in lactate production but negligible oxidation of pyruvate in the Krebs cycle. Hyperpolarization methods offer a novel approach to studying lactate and pyruvate metabolism in human skeletal muscle that is readily acceptable to subjects.
Introduction
Lactate metabolism plays a central role in exercise physiology. Metabolism of glucose to pyruvate (glycolysis) may be followed by pyruvate oxidation in the Krebs cycle, or by export of lactate from muscle. This process provides for rapid ATP production, and the accumulation of lactate in muscle correlates with fatigue. Invasive measurements of lactate in skeletal muscle are undesirable and for this reason 1H NMR methods have been applied. Because of proximity to the water signal, chemical exchange methods to detect the hydroxyl proton and direct detection of H2 are both difficult, and the methyl protons overlap with lipids. Hyperpolarization methods offer the possibility of simultaneously measuring both fates of pyruvate, oxidation for energy production and generation of lactate. In skeletal muscle, pyruvate may be oxidized via pyruvate dehydrogenase to CO2 and acetyl-CoA for oxidation in the Krebs cycle, or converted to lactate via lactate dehydrogenase, thereby consuming NADH and enabling continuation of glycolysis. In this study, pyruvate metabolism in skeletal muscle was studied in healthy human subjects using hyperpolarized [1-13C]pyruvate at rest and after toe-lift exercise.Methods
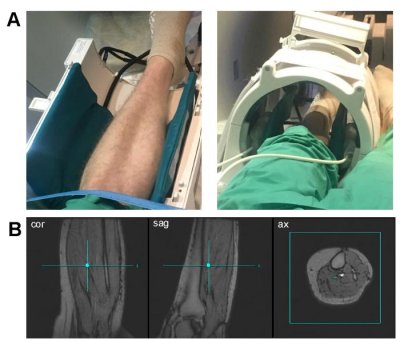
Two healthy volunteers (27 years old male, 65 years old female) were recruited for the study. Hyperpolarized [1-13C]pyruvate was prepared as previously described [1,2]. Calf skeletal muscle of each subject’s left leg was positioned at the center of 13C/1H dual-frequency head coil (quadrature Tx/Rx for 1H, quadrature Tx/8-channel array Rx for 13C, Fig.1) [3]. Subjects were received two injections of hyperpolarized pyruvate with time interval of 30 min between the injections. After 1H localizer and shimming, the first hyperpolarized [1-13C]pyruvate was administered intravenously, immediately followed by a slice-selective 13C MR spectroscopy (flip angle = 5o, slice thickness = 10 cm, TR = 3 sec, scan time = 4 min). The subjects were asked not to use their leg muscle extensively at least for 1 hr prior to the first injection to maintain a resting-state. After the first pyruvate injection and approximately 5 min prior to the second injection, the subjects performed a one-leg toe-lifting exercise using a wooden panel (1 min, a lift every 2 sec, ~30 lifts total). The exercise calf muscle was repositioned similar to the first 13C scan.Results and Discussion
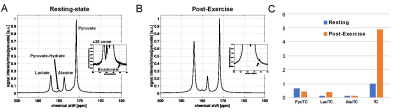
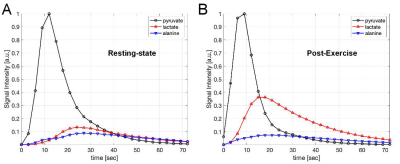
Lactate and alanine are predominantly produced from hyperpolarized 13C-pyruvate in the skeletal muscle both at pre- and post-exercise. Although the polarization levels of pyruvate samples were comparable between the injections, the total 13C signals (TC) detected by the coil was increased by 4.87-times (x4.92 for subject#1, x4.82 for subject#2), which is probably due to the increased perfusion to the exercised tissue. Another noticeable change was the amount of lactate produced; [1-13C]lactate, normalized by TC, increased from 0.16 to 0.40 due to the anaerobic exercise. Accordingly, [1-13C]pyruvate/TC and [1-13C]alanine/TC were reduced from 0.67 to 0.47 and 0.16 to 0.14, respectively, after the exercise. [13C]Bicarbonate was just above the noise level, but not accurately quantifiable at the resting-state, and became more visible post-exercise due to the increased perfusion (Fig.2). From both subjects, lactate and alanine peaked earlier by 3-6 sec after exercise, compared to the time curves at resting state (Fig.3). We plan to perform statistical analysis as well as kinetic analysis with more subjects.Conclusion
The in vivo results suggest that the hyperpolarized 13C-pyruvate is capable to assess a cellular shift from aerobic to anaerobic condition and O2 insufficiency. We expect that the technique can be utilized to study metabolic disorders associated with carbohydrate metabolism in skeletal muscle such as glycogen storage diseases (e.g., McArdle disease).Acknowledgements
The Texas Institute of Brain Injury and Repair; National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award.References
[1] S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, P. E. Z. Larson, A. L. Harzstark, M. Ferrone, M. Van Criekinge, J. W. Chang, R. Bok, I. Park, G. Reed, L. Carvajal, E. J. Small, P. Munster, V. K. Weinberg, J. H. Ardenkjaer-Larsen, A. P. Chen, R. E. Hurd, L.-I. Odegardstuen, F. J. Robb, J. Tropp, and J. A. Murray, “Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate.,” Sci Transl Med, vol. 5, no. 198, p. 198ra108, Aug. 2013.
[2] J. M. Park, J. Liticker, C. Harrison, G. Reed, T. Hever, J. Ma, R. Martin, D. Mayer, R. Hashoian, C. Madden, M. Pinho, and C. Malloy, “Feasibility and reproducibility of imaging brain metabolism using hyperpolarized 13C pyruvate in humans.,” ISMRM. #4311, 2019.
[3] J. Ma, R. Hashoian, C. Sun, S. Wright, A. Ivanishev, R. Lenkinski, C. Malloy, A. Chen, and J. M. Park, “Development of 1H/13C RF head coil for hyperpolarized 13C imaging of human brain.,” ISMRM. #568, 2019.
Figures