2998
Dynamic Oxygen-17 MRI with Adaptive Reconstruction using Golden-Means-Based 3D Radial Sampling1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Case Cardiovascular Research Institute, Case Western Reserve University, Cleveland, OH, United States, 3Department of Neurology, Case Western Reserve University, Cleveland, OH, United States, 4Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 5Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
A golden-means-based 3D radial sampling and k-space weighted image reconstruction method was developed for dynamic tracking of intravenously injected 17O-water in mouse brain. The method enabled image reconstruction with adaptive temporal resolution of 3 to 15 s to capture the regional differences in 17O-water uptake and washout kinetics in post-stroke mice, with an isotropic voxel size of 1.77 µL on a 9.4T scanner.
Introduction
Dynamic 17O-MRI enables imaging of cerebral perfusion and metabolism. However, current 17O-MRI methods offer limited spatial and temporal resolution due to the low sensitivity and fast T2 decay of 17O signal. Imaging strategy using stack-of-stars sampling and k-space view-sharing has enabled dynamic tracking of intravenously injected 17O-water in mouse brain with 7.6-s temporal resolution and 11.25-μL spatial resolution.1 However, further improving the spatial resolution using this approach entails increasing the phase-encoding steps, leading to reduced temporal resolution. In the current study, we employed golden-means-based 3D radial sampling to enable flexible reconstruction of dynamic 17O-MRI data with isotropic spatial resolution and adaptive temporal resolution tailored to the dynamics of the acquired signal. The efficacy of this method was demonstrated in quantifying 17O-water uptake and washout kinetics in post-stroke mouse brain.Methods
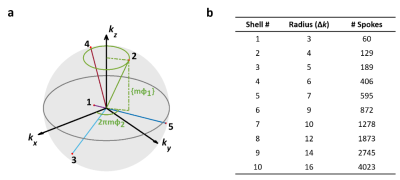
3D Radial Sampling: All MRI studies were performed on a Bruker 9.4T scanner. 17O data were acquired with a 2-cm surface coil. Data acquisition used center-out, golden-means-based 3D radial sampling proposed by Chan et al (Fig. 1a).2 Specifically, for the mth spoke, its azimuth angle and projection on the z-axis were $$$2\pi m\varphi_1$$$ and $$$(-1)^mk_{max}mod(m\varphi_2,1)$$$, respectively, with $$$\varphi_1$$$ (=0.6823) and $$$\varphi_2$$$ (=0.4656) being the 2D golden means derived from the Fibonacci matrix. This sampling scheme achieved a nearly uniform coverage of k-space with an arbitrary number of spokes. For each spoke, 16 data points were acquired in 2 ms with 24x24x24 mm3 FOV, yielding a nominal isotropic resolution of 0.46 μL. TR and TE were 10 ms and 75 μs, respectively.Image Reconstruction: Image reconstruction used a 3D k-space weighted image contrast (KWIC) strategy for simultaneous high temporal and spatial resolution.3 For a specific time frame, the k-space was partitioned into a set of concentric shells. Each shell consisted of radial spokes acquired around the time frame of interest, with the number of spokes being a Fibonacci number to ensure the most uniform coverage. The minimal number of spokes needed to fulfill the Nyquist criterion in each shell is shown in Fig 1b. With the center k-space partition updated every 60 spokes, this sampling and reconstruction scheme gave rise to an effective temporal resolution of 0.6 s. Further, temporal resolution can be traded to gain SNR by increasing the number of spokes in each shell.
Simulation: Simulation studies were performed on a modified Shepp-Logan phantom to optimize the data reconstruction strategy. The phantom consisted of three compartments, with three segments in the time courses of 17O signal changes in each compartment representing the baseline, the injection, and the washout phases of an 17O-water injection experiment (Fig. 2a). Gaussian noise was added to the k-space data at a level similar to in vivo SNR. Image reconstruction used varied temporal resolution. A 3-s window size was used to capture the rapid signal increase during the injection phase, and it transitioned from 3 to 15 s during the washout phase to achieve the best trade-off between SNR and accurate delineation of signal dynamics (Fig. 2b). Parameter estimation using the adaptive reconstruction strategy was compared with that using fixed window size of 3 s and 15 s.
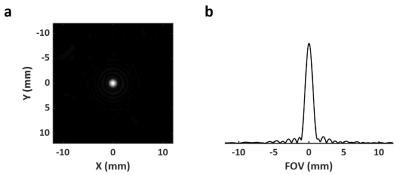
Point Spread Function: Point spread function (PSF) using the proposed acquisition and reconstruction strategy was measured on a point-source phantom. A 1-mm microhematocrit capillary tube filled with 17O-water (40% enrichment) was placed in parallel to the z-direction of the magnet. The full width half maximum (FWHM) of the PSF was used to calculate the actual voxel size.
In Vivo Study: Adult male C57BL/6 mice (n=8) underwent 60-min middle cerebral artery occlusion (MCAO) surgery. MRI scan was performed 2 hours post MCAO with 0.1 µL of 17O-water injected via tail vein. 17O-MRI acquisition was performed continuously for 25 min covering baseline (8 min), injection (~10 s), and washout (17 min) phases. Peak and steady-state 17O signal, and the washout rate of 17O-water was estimated.
Results
Point Spread Function: Fig. 3a shows the cross-sectional image of a microhematocrit capillary tube. Its 1D profile was considered representative of the PSF reconstructed using the proposed method. The FWHM of the PSF was 1.21 mm, corresponding to a true voxel size of 1.77 µL.Simulation Study: Simulation results are shown in Fig. 2c-e. The 17O signal changes in each compartment was representative of 17O-water kinetics in normal (#1), infarct core (#2), and penumbra tissue (#3) (Fig. 2a). The window size for adaptive reconstruction was derived from the derivative of the center k-space data (Fig. 2b). Comparing to reconstruction with fixed window sizes, adaptive reconstruction led to more accurate parameter estimation with reduced deviation from the ground truth (Fig. 2c-e).
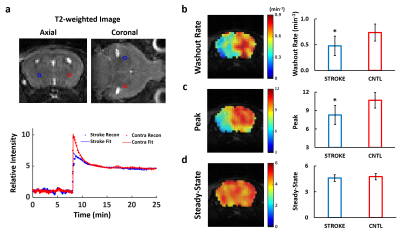
In Vivo Study: Fig. 4 compares 17O dynamics in MCAO-affected and contralateral regions in post-stroke mouse brain. Slower transition from wash-in phase to wash-out phase was observed in the stroke-affected region (Fig. 4a). Peak 17O-water uptake and washout rate were reduced by 20% and 30%, respectively, in stroke-affected hemisphere, suggesting a perfusion deficit.
Discussion and Conclusion
3D golden-means-based radial sampling, in combination with KWIC reconstruction, enables reconstruction of 17O-MRI data with adaptive window size for accurate tracking of the dynamics of 17O-water with high isotropic spatial resolution in post-stroke mouse brain.Acknowledgements
This work was supported by a grant from the National Institute of Health (R01 EB23704).References
1. Liu Y, Zhang Y, Wu C, Zhu J, Wang C, Tomko N, Linetsky MD, Salomon RG, Ramos-Estebanez C, Wang Y, Yu X. High-resolution dynamic oxygen-17 MR imaging of mouse brain with golden-ratio–based radial sampling and k-space–weighted image reconstruction. Magn Reson Med. 2018;79:256–263.
2. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal Stability of Adaptive 3D Radial MRI Using Multidimensional Golden Means. Magn Reson Med. 2009;363:354–363.
3. Song HK, Dougherty L. k-Space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000;44:825–832.
Figures