2992
Detection of Human Muscle Glycogen by Natural Abundance Carbon-13 MRS at 7 T with Short Duration Proton Decoupling1National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States, 2National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
In this work, a continuous wave (CW) proton decoupling with a short duration of 15 ms was used to detect natural abundance 13C glycogen C1 signals in the human calf at 7 T. This short duration CW proton decoupling dramatically reduced RF power deposition, which allowed the TR to be shortened to 310 ms and additional NOE pulses to be used to ensure optimal SNR performance. The reconstructed glycogen spectra and the peak area ratio between decoupled and non-decoupled glycogen C1 resonances demonstrated that adequate proton decoupling was achieved.
INTRODUCTION
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in human body. It is a ubiquitous fuel source stored in the cytosol of cells, occupying 1%–2% of the volume of skeletal muscle cells. Glycogen levels in skeletal muscles may change dramatically in several diseases such as McArdle disease (MD), phosphofructokinase deficiency (PFKD), and acid maltase deficiency (AMD). Natural abundance 13C magnetic resonance spectroscopy (MRS) was first used to detect glycogen in the human calf muscle at 4.7 T1. The pulse sequence used a TR of 205.8 ms; proton decoupling was turned on for the 20 ms acquisition period; and no pulses for the Nuclear Overhauser effect (NOE) were used. In the following years, glycogen has been measured at different field strengths such as 1.5 T, 3 T, and 4 T. However, none of these studies used proton decoupling and NOE together due to specific absorption rate (SAR) limits. In the studies that used proton decoupling, the duration of decoupling was longer than 40 ms. In a recent work2, 13C MRS measurement of muscle glycogen was performed at 7 T using a pulse sequence with a TR of 800 ms, 50 ms WALTZ-16 proton decoupling, and no NOE. Even though decoupling generates partial NOE, additional NOE pulses are useful to enhance SNR with acceptable SAR. We propose to use a continuous wave (CW) proton decoupling with a short duration of 15 ms for natural abundance 13C MRS of muscle glycogen at 7 T. This short duration CW proton decoupling dramatically reduces RF power deposition at 7 T and thus allows the TR to be shortened and additional NOE pulses to be used to ensure maximum NOE.METHODS
All experiments were performed using a Siemens Magnetom 7 T scanner in combination with an in-house built RF coil assembly comprised a circular 13C coil (diameter = 7 cm) and a quadrature half-volume proton coil. The pulse sequence used a rectangular excitation pulse of 90° flip angle and 100 ms duration. Data acquisition had a duration of 102.4 ms and CW proton decoupling was turned on during the first 15 ms of the acquisition period. The decoupling pulse had a B1 of 290 Hz and its frequency was tuned to the glycogen C1 resonances at 5.39 ppm. Additional rectangular-shaped NOE pulses with a duration of 1 ms and B1 of 330 Hz were applied for every 50 ms during the T1 relaxation period. For a 310 ms TR and 5800 averages, the total scan time was 30 min. The time averaged RF power was 5.9 W when both decoupling and NOE were applied.RESULTS
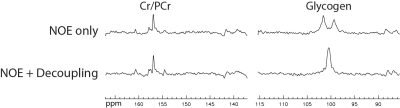
The spectra acquired from the left calf of a 16-year-old male subject with NOE only and with both NOE and decoupling are displayed in Figure 1. Two segments of each spectrum containing glycogen and creatine/phosphocreatine resonances are displayed. We can see that the glycogen doublet without decoupling collapsed into a singlet with decoupling. The glycogen peak area ratio between the spectra acquired with and without decoupling was found to be 0.93. This meant that sideband signal loss due to decoupling was very small and adequate decoupling had been achieved.DISCUSSION AND CONCLUSION
In a previous study3, the in vitro T1 values of glycogen were found to be 65 ± 5 ms at 2.1 T, 142 ± 10 ms at 4.7 T, and 300 ± 10 ms at 8.4 T. By interpolation, we estimated that glycogen T1 was ~250 ms at 7 T. Using a 90° excitation pulse, TR of 310 ms offered maximum SNR efficiency. In the same study3, the in vitro T2 values of glycogen were found to be 9.4 ± 1 ms at 4.7 T, and 9.5 ± 1 ms at 8.4 T, which were virtually identical. The in vivo glycogen T2 of rodents was found to be 5 ± 2 ms at 4.7 T, which is significantly shorter than the reported in vitro values. Based on the above reports, the in vivo T2 of glycogen at 7 T is close to 5 ms, which indicates that 15 ms proton decoupling should be sufficient. The spectra displayed in Figure 1 and their peak area ratio demonstrated that a 15 ms CW pulse with B1 = 290 Hz provided adequate proton decoupling at 7 T. This short duration proton decoupling reduced RF power deposition, which allowed a TR of 310 ms and additional NOE pulses to ensure optimal SNR performance for 13C measurement of natural abundance glycogen at 7 T.Acknowledgements
This work was supported by the intramural programs of the NIH.References
1. Avison MJ, Rothman DL, Nadel E, Shulman RG. Detection of human muscle glycogen by natural abundance 13C NMR. Proc Natl Acad Sci U S A 1988;85(5):1634-1636.
2. Heinicke K, Dimitrov IE, Romain N, Cheshkov S, Ren JM, Malloy CR, Haller RG. Reproducibility and Absolute Quantification of Muscle Glycogen in Patients with Glycogen Storage Disease by C-13 NMR Spectroscopy at 7 Tesla. Plos One 2014;9(10).
3. Zang LH, Laughlin MR, Rothman DL, Shulman RG. 13C NMR relaxation times of hepatic glycogen in vitro and in vivo. Biochemistry 1990;29(29):6815-6820.
Figures