2990
Application of a BIlinear Rotation Decoupling (BIRD) filter for indirect 13C measurements using J-difference editing in the liver1Departments of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Nutrition and Movement Sciences, Maastricht University Medical Center, Maastricht, Netherlands
Synopsis
Indirect 13C measurements provide a large signal gain when compared to direct 13C-MRS. The regular J-difference editing (JDE) techniques are quite challenging to apply in the liver, as respiratory motion will result in imperfect subtraction of the huge overlapping 1H-[12C] signal, which hampers accurate quantification of the 1H-[13C] signals. Here, we demonstrated that the application of a BIlinear Rotation Decoupling filter prior to a regular JDE (STEAM-ACED) to pre-suppress the hindering 1H-[12C] signal, leads to a robust detection of the 1H-[13C] signal irrespective of respiratory motion. We showed an application of measuring 1H-[13C] lipid signals from the liver in vivo.
Introduction
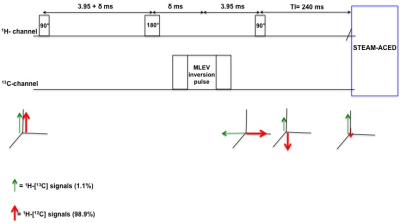
Indirect 13C-MRS (i.e. the detection of 1H signals that are coupled to 13C-nuclei) leads to a large signal gain when compared to direct 13C-MRS. Recently, we demonstrated that it is possible to noninvasively follow the retention of labeled 13C-substrate into the hepatic lipid pool in vivo using gradient enhanced heteronuclear single quantum coherence (ge-HSQC) spectroscopy1. ge-HSQC was chosen in these measurements, as the use of regular J-difference editing (JDE) approaches2 results in imperfect subtraction and thereby incomplete suppression of the (large) overlapping 1H-[12C] lipid signal in vivo due to respiratory motion. However, the drawback of applying ge-HSQC is that it inherently leads to a signal loss of 50% when compared to JDE sequence (e.g. STEAM localization with adiabatic carbon editing and decoupling, STEAM-ACED). Here, we hypothesize that pre-suppression of the strong 1H-[12C] lipid signal, by introducing a BIlinear Rotation Decoupling (BIRD) filter3 prior to a STEAM-ACED4 sequence, optimized for detection of 1H-[13C] lipid signals, will lead to an accurate and robust detection of the 1H-[13C] lipid signal irrespective of respiratory motion, with higher signal yield as compared to ge-HSQC.Materials and methods
All experiments were performed on a 3T MR system (Achieva 3T-X Philips Healthcare) using a double tuned 1H/13C surface coil in quadrature mode (Rapid Biomedical GmbH). 1H-[13C] lipid spectra were acquired from a peanut oil phantom, with three different MR sequences: ge-HSQC, STEAM-ACED and the STEAM-ACED sequence with additional BIRD filter (BIRD-STEAM-ACED). Power calibration was performed initially by acquiring a series of 1H-lipid spectra with different flip angles. Firstly, ge-HSQC was used to acquire 1H-[13C] lipid spectra with t1=4 ms, TR/TE=4000/7.9 ms, NSA=128, voxel=30x30x30mm, datapoints=2048. In a second experiment, spectra were acquired with a STEAM-ACED sequence using identical parameters as from the ge-HSQC. An adiabatic 13C inversion pulse (bandwidth=1600 Hz) was used on alternate scans for editing purpose. In a third experiment, the BIRD filter was inserted prior to the STEAM-ACED sequence (figure 1) and an inversion time (TI) of 240 ms was chosen to selectively pre-suppress the 1H-[12C] lipid signal. Additionally, the efficiency of the BIRD filter was tested in vivo in a volunteer with a fatty liver (age-26, BMI-28 Kg/m2, liver fat-14%). A regular STEAM-ACED spectrum was acquired from a voxel of 45x45x45 mm and compared with spectrum of the BIRD-STEAM-ACED. The TR of 4000 ms was chosen to allow the subject to breathe in the rhythm of the measurement and MR acquisition performed during expiration. All acquired spectra were post-processed using a custom written MATLAB script.Results and discussion
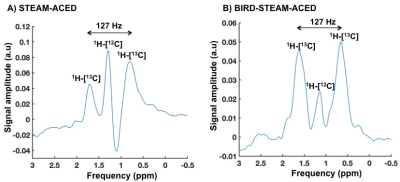
The subtraction artifact of the 1H-[12C] lipid signal was clearly visible even in the phantom when a conventional STEAM-ACED sequence was used (figure 2B). Due to the implementation of the BIRD filter, the subtraction artifact was largely reduced and therefore we were able to visualize the target 1H-[13C]-lipid signals clearly as two distinct peaks with known coupling constant of 127 Hz (figure 2C). Moreover, the 13C lipid signal as measured with the BIRD-STEAM-ACED was approximately 2-fold higher when compared with the ge-HSQC (figure 2A & 2C). Currently, we are working on a protocol to determine the 13C-enrichment (natural abundance) from these spectra.Similarly, as expected, a large subtraction artifact was apparent in in vivo when a STEAM-ACED sequence was used (figure 3A). This artifact leads to phase distortions in the edited spectrum, which will hamper the accurate and robust quantification of the 1H-[13C] lipid signal. The addition of the BIRD-filter leads to pre-suppression of the huge 1H-[12C]-lipid signal during acquisition and thereby leads to marked reduction in the subtraction artifact. (figure 3B).
Conclusion
Our proposed scheme with the addition of a BIRD filter reduces the subtraction artifact efficiently in JDE experiments, which leads to a signal gain of 100% as compared to the previously applied method (ge-HSQC). Thus, the BIRD-STEAM-ACED sequence can now be used for 13C-tracking experiments in the human liver in vivo.Acknowledgements
This research was in part financed by the Ministry of Economic Affairs and Climate Policy by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships and by Unilever R&D Vlaardingen. LL was supported by a Veni grant from ZonMW (016.Veni.188.036)References
1. Lindeboom L, De Graaf R.A, Nabuurs C.I, et al. Quantum coherence spectroscopy to measure dietary fat retention in the liver. JCI Insight. 2016;1(13):e84671.
2. De Graaf R.A, Rothman D.L, and Behar K.L. State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo: A practical guide. NMR Biomed.2011;24(8):958-972.
3. Garbow J.R, Weitekamp D.P, and Pines A. Bilinear rotation decoupling of homonuclear scalar interactions. Chem. Phys. Lett.1982;93(5):504-509.
4. Pfeuffer J, Tkac I, Choi I-Y, et al. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn. Reson. Med.1999;41(6):1077-1083.
Figures